Difference between revisions of "UW-Stout/Hydrogen Peroxide SP22"
(→Equipment) |
|||
| (38 intermediate revisions by the same user not shown) | |||
| Line 10: | Line 10: | ||
*Wild Type and Knockout Yeast Strains | *Wild Type and Knockout Yeast Strains | ||
| − | + | #BY4735 | |
| + | #[[YBL113C]] | ||
| + | #[[YCL049C]] | ||
| + | #[[YDL109C]] | ||
| + | #[[YDR307W]] | ||
| + | #[[YGL146C]] | ||
| + | #[[YGR079W]] | ||
| + | #[[YHL029C]] | ||
| + | #[[YNL058C]] | ||
| + | #[[YOR111W]] | ||
==Equipment== | ==Equipment== | ||
| Line 18: | Line 27: | ||
*P-10 Micropipette | *P-10 Micropipette | ||
*Centrifuge Tubes | *Centrifuge Tubes | ||
| − | * | + | *Two Small Beakers |
| + | *Lab Plate With Wells | ||
==Calibration Protocol== | ==Calibration Protocol== | ||
| − | H2O2 Percent | + | ===H2O2 Dilutions=== |
| + | |||
| + | # Vortex yeast strains | ||
| + | # Create 50ul Hydrogen Peroxide dilutions as required | ||
| + | # Mix dilutions With 50ul yeast strains | ||
| + | # Place mixtures in individual wells on a lab plate to grow for 24 hours | ||
| + | |||
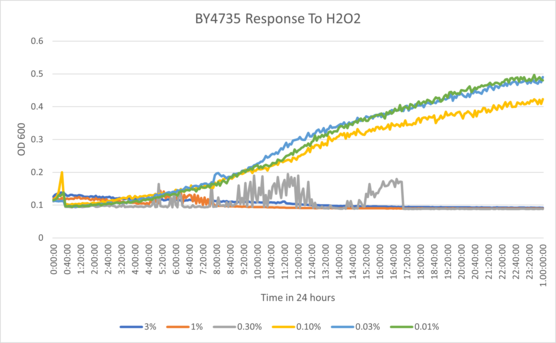
| + | ===Calibration Trial Percent Series: 3%, 1%, 0.3%, 0.1%, 0.03%, 0.01%=== | ||
| − | |||
| − | + | [[File:revise1trial.png]] | |
| − | |||
| − | |||
| + | ===Interpretation=== | ||
| + | Based on the calibration experiment, we determined that the concentration of hydrogen peroxide tested on the yeast would need to be much lower if we wanted to see consistent amounts of inhibition and compare them. The higher concentrations in the experiment yielded data was too close among the wells, so based on the calibration we settled on a dilution of 0.065%, as the range of 0.03% to 0.1% seemed to show the most observable differences in data. | ||
| + | ==Knockout Protocol== | ||
| − | + | ===Procedure=== | |
| − | + | # Vortex yeast strains | |
| − | + | ##BY4735 | |
| + | ##[[YBL113C]] | ||
| + | ##[[YCL049C]] | ||
| + | ##[[YDL109C]] | ||
| + | ##[[YDR307W]] | ||
| + | ##[[YGL146C]] | ||
| + | ##[[YGR079W]] | ||
| + | ##[[YHL029C]] | ||
| + | ##[[YNL058C]] | ||
| + | ##[[YOR111W]] | ||
| + | # Create a 50ul Hydrogen Peroxide dilution for a final solution of 0.065% concentration (one for each strain) | ||
| + | # Mix dilutions With 50ul yeast strains | ||
| + | # Place mixtures in individual wells on a lab plate to grow for 24 hours | ||
| + | # Place standard controls of each strain (50ul yeast mixed with 50ul of water) in individual wells on a lab plate to grow for 24 hours as comparison | ||
| + | # Plot growth data. | ||
Latest revision as of 19:30, 4 May 2022
Contents
Introduction
Hydrogen Peroxide (H2O2) is a very strong oxidizer and antiseptic that is often used in dilute forms. The overall compound is found in biological systems but is in short lived reactions due to it's high toxicity over time. The goal of our experiment was to test how multiple yeast strains with knockout genes would respond in the presence of varying concentrations of hydrogen peroxide.
Materials
- Hydrogen Peroxide Solution 3% Concentration
- Distilled water for dilutions
- Wild Type and Knockout Yeast Strains
Equipment
- P-200 Micropipette
- P-20 Micropipette
- P-10 Micropipette
- Centrifuge Tubes
- Two Small Beakers
- Lab Plate With Wells
Calibration Protocol
H2O2 Dilutions
- Vortex yeast strains
- Create 50ul Hydrogen Peroxide dilutions as required
- Mix dilutions With 50ul yeast strains
- Place mixtures in individual wells on a lab plate to grow for 24 hours
Calibration Trial Percent Series: 3%, 1%, 0.3%, 0.1%, 0.03%, 0.01%
Interpretation
Based on the calibration experiment, we determined that the concentration of hydrogen peroxide tested on the yeast would need to be much lower if we wanted to see consistent amounts of inhibition and compare them. The higher concentrations in the experiment yielded data was too close among the wells, so based on the calibration we settled on a dilution of 0.065%, as the range of 0.03% to 0.1% seemed to show the most observable differences in data.
Knockout Protocol
Procedure
- Vortex yeast strains
- Create a 50ul Hydrogen Peroxide dilution for a final solution of 0.065% concentration (one for each strain)
- Mix dilutions With 50ul yeast strains
- Place mixtures in individual wells on a lab plate to grow for 24 hours
- Place standard controls of each strain (50ul yeast mixed with 50ul of water) in individual wells on a lab plate to grow for 24 hours as comparison
- Plot growth data.