UW Stout/Nystatin SP22
Contents
Introduction
Through the following calibration and knockout protocols, we will be stressing different strains of yeast cells with Nystatin.
Materials
- Nystatin Powder
- Dimethylformamide(DMF)
- Sterile Water
- 1.7ml tubes
- Weighing boat
- [COSTAR 96-well clear flat-bottom assay plate]
- Glycerol stock of wild type and knockout yeast strains as prepared in steps 1-4 of the [growth curve protocol]
Equipment
- Vortex
- Scale
- [Devices SpectraMax Plus 384 Microplate Reader]
Calibration Protocol
An excess amount of each concentration was made incase of errors during plating
Precautions: We used gloves and lab coats throughout these protocols. Handle DMF with caution, as it can cause skin irritation. Wash hands immediately if DMF comes in contact with skin.
Trial 1
- Dissolve Nystatin in DMF:
- Use scale and weighing boat to measure 40mg of Nystatin powder
- Add Nystatin to a tube
- Measure and add 10 mL DMF to the same tube
- Mix gently until no powder is present
- Mix the dissolved Nystatin with sterile water to get the following amounts of Nystatin per 50µl of solution
- 0µg: 0µl Nystatin, 50µl H2O
- 10µg: 25µl Nystatin, 475µl H2O
- 20µg: 50µl Nystatin, 450µl H2O
- 30µg: 75µl Nystatin, 425µl H2O
- 40µg: 100µl Nystatin, 400µl H2O
- 50µg: 125µl Nystatin, 375µl H2O
- 60µg: 150µl Nystatin, 350µl H2O
- 70 µg: 175 Nystatin, 325µl H2O
- Vortex yeast culture briefly to resuspend yeast cells
- Set up 8 wells in the assay plate, each with 50µl of wild type yeast culture and 50µl of solution, using one solution per well
- Set up the plate reader:
- Temperature: 30°C
- Mode: Kinetic
- Read: 600nm
- Interval: 5 minutes
- Total run time: 24 hours
- Shake before read: 30 seconds
- Transfer the plate to the reader and run for 24 hours
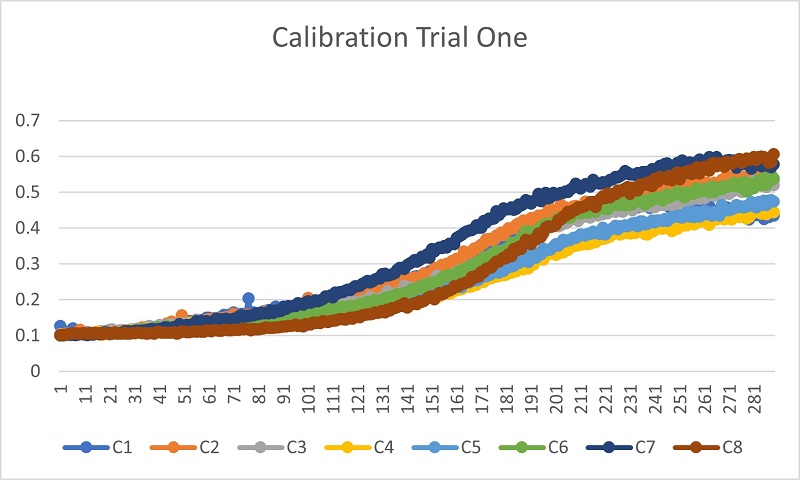
Trail 1 Data
Trial 1 showed that the concentration of the stressor was too high. Though the growth of the yeast cells was affected, it was due to the high amount of the solvent DMF, which killed the yeast cells.
Trial 2
- Use the dissolved Nystatin from step 1 of trial one to create a first dilution:
- 1µl initial 4µg/µl stock, 999µl H2O (final concentration 4µg/ml)
- Mix the first dilution stock with sterile water to get the following amounts of Nystatin per 50µl of solution
- 0µg/ml or 0µg/l: 200ul water
- .5 µg/ml or .0005 µg/µl: 25µl stock, 175µl H2O
- .75 µg/ml or .00075 µg/µl: 37.5µl stock, 162.5µl H2O
- 1.0 µg/ml or .001 µg/µl: 50µl stock, 150µl H2O
- 1.25 µg/ml or .00125 µg/µl: 62.5µl stock, 137.5µl H2O
- 1.5 µg/ml or .0015 µg/µl: 75µl stock, 125µl H2O
- 1.75 µg/ml or .00175 µg/µl: 87.5µl stock, 112.5µl H2O
- 2.0 µg/ml or .002 µg/µl: 100µl stock, 100µl H2O
- Repeat steps 3-6 from Trial 1
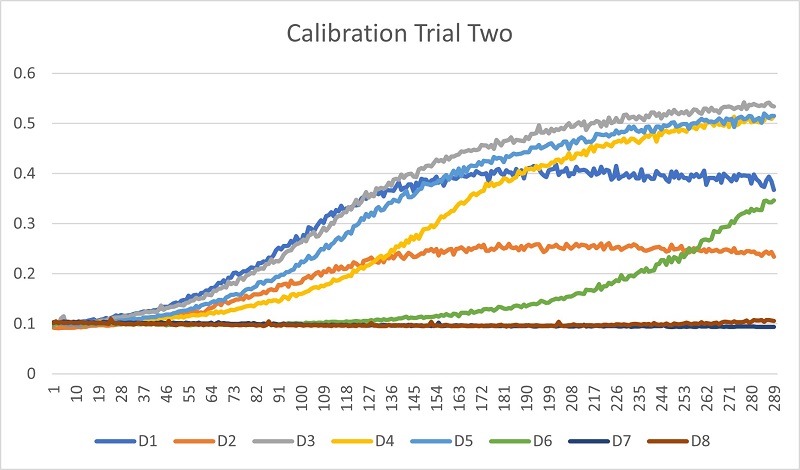
Trial 2 Data
Trial 2 showed that the yeast cells were growing, but the Nystatin was not concentrated enough to have an effect.
Trial 3
- Use the dissolved Nystatin from step 1 of trial one to create a first dilution:
- 4µl initial 4µg/µl stock, 996µl H2O (final concentration 16µg/ml)
- Mix the first dilution stock with sterile water to get the following amounts of Nystatin per 50µl of solution
- 0µg/ml or 0µg/µl: 200µl water
- 2µg/ml or .002µg/µl: 25µl stock, 175µl H2O
- 3µg/ml or .003µg/µl: 37.5µl stock, 162.5µl H2O
- 4µg/ml or .004µg/µl: 50µl stock, 150µl H2O
- 5µg/ml or .005µg/µl: 62.5µl stock, 137.5µl H2O
- 6µg/ml or .006µg/µl: 75µl stock, 125µl H2O
- 7µg/ml or .007µg/µl: 87.5µl stock, 112.5µl H2O
- 8µg/ml or .008µg/µl: 100µl stock, 100µl H2O
- Repeat steps 3-6 from Trial 1
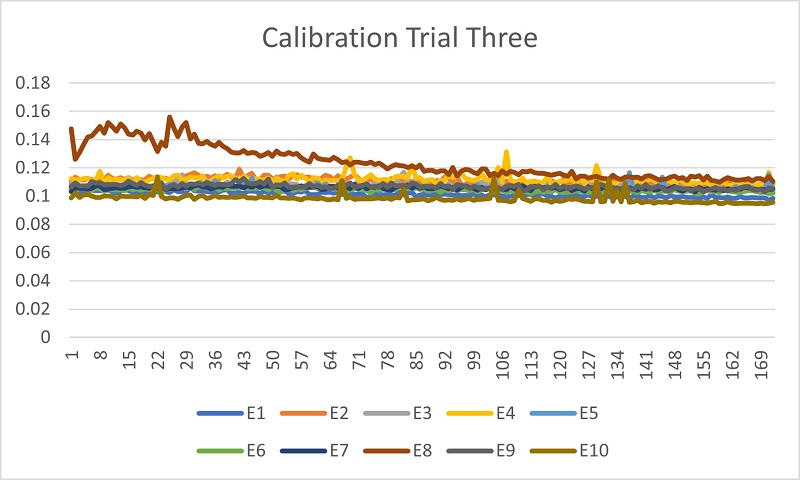
Trial 3 Data
Trial 3 showed that the concentration of Nystatin was high enough to have an effect. We chose the 1µg/ml concentration for our final experiment. This concentration was enough to inhibit cell growth, but doesn’t completely stop the cells from growing.
Knockout Protocol
Use the data from the Calibration Protocol for the optimal concentration of Nystatin
- Vortex yeast culture briefly to resuspend yeast cells
- Set up 10 wells in the assay plate, each with 50µl of stock and 50µl of yeast cells, using one strain in each well.
- Set up the plate reader:
- Temperature: 30°C
- Mode: Kinetic
- Read: 600nm
- Interval: 5 minutes
- Total run time: 24 hours
- Shake before read: 30 seconds
- Transfer the plate to the reader and run for 24 hours
Note:Repeat the protocol to account for natural variation
Knockout Protocol Results
- Knock-out Strain Wild Type
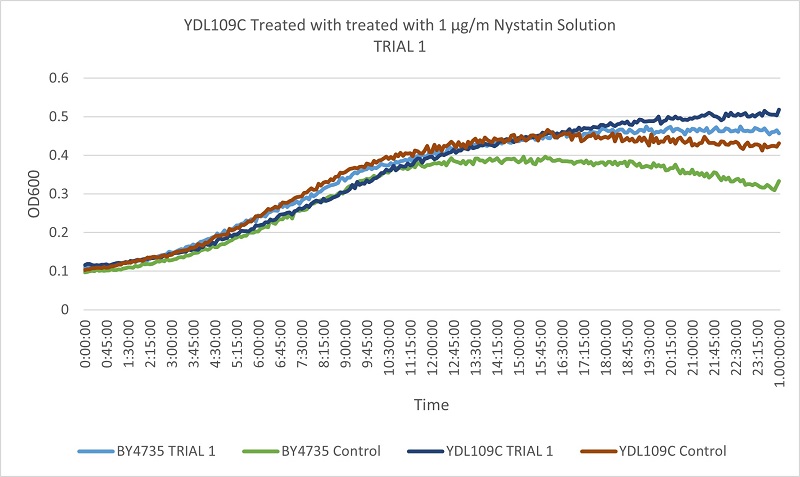
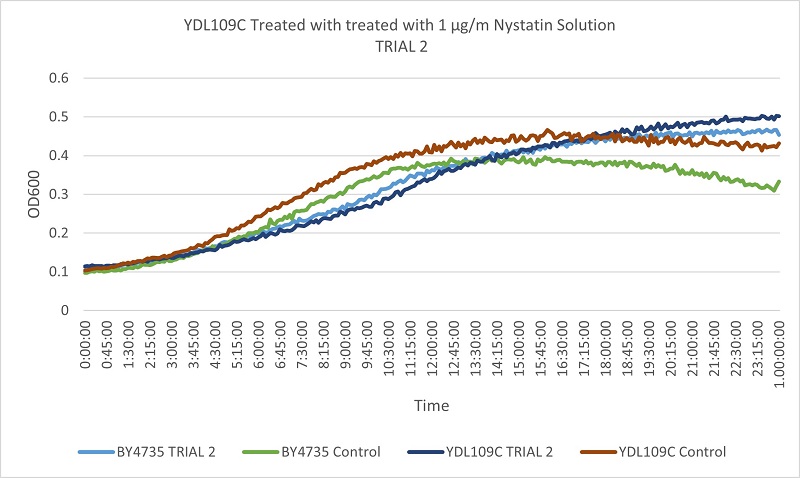
- Knock-out Strain 1:YDL109C
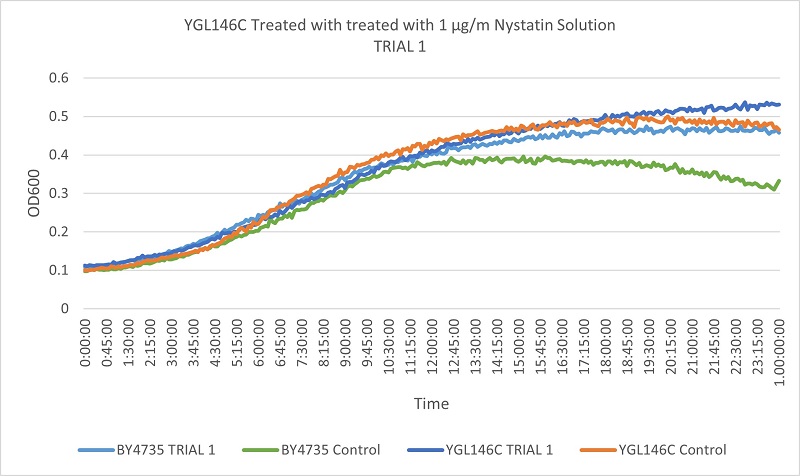
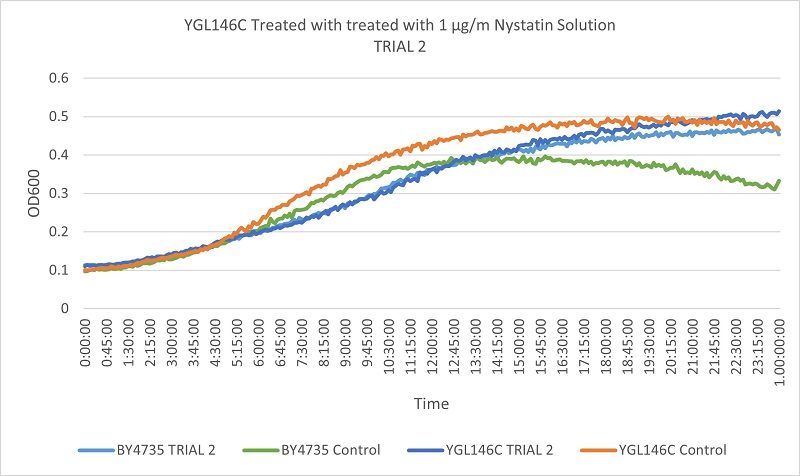
- Knock-out Strain 2:YGL146C
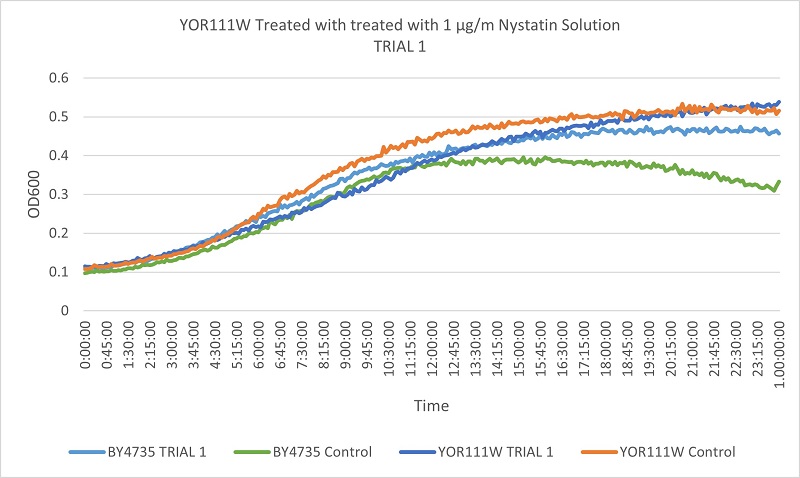
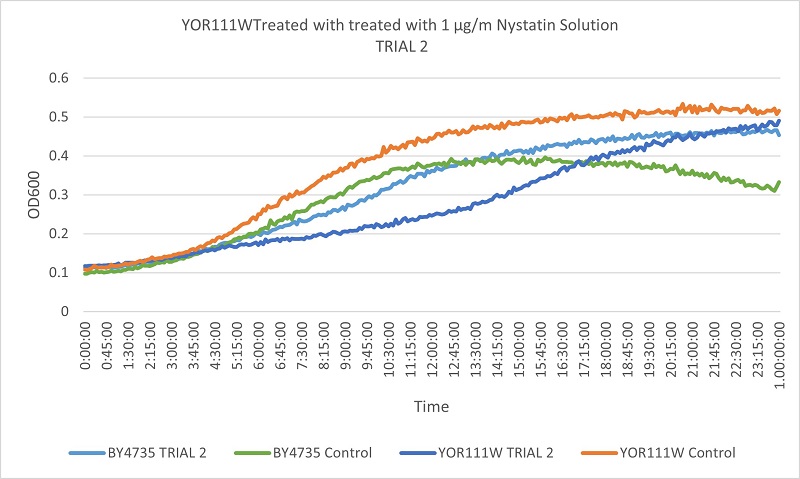
- Knock-out Strain 3:YOR111W
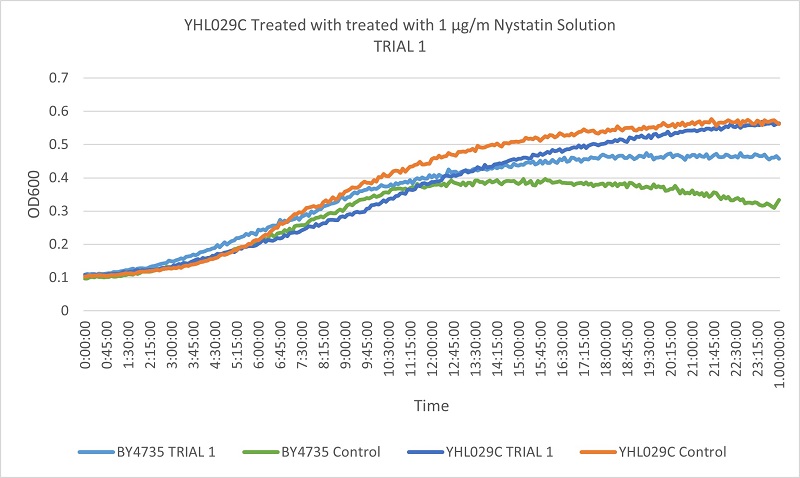
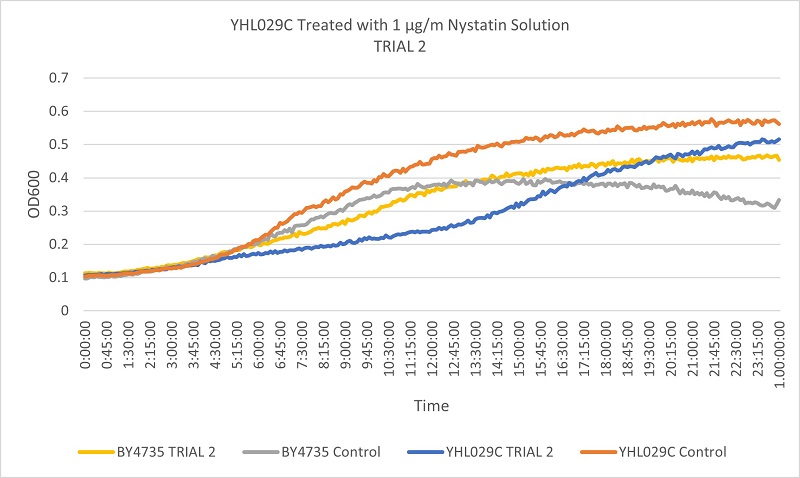
- Knock-out Strain 4:YHL029C
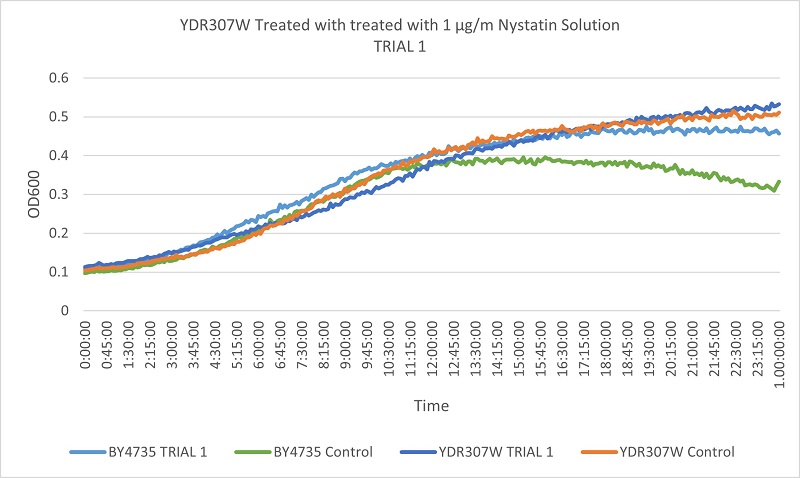
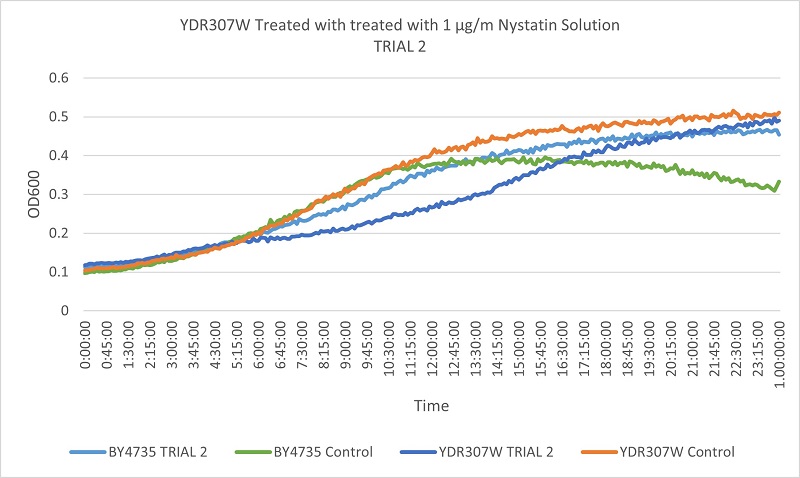
- Knock-out Strain 5:YDR307W
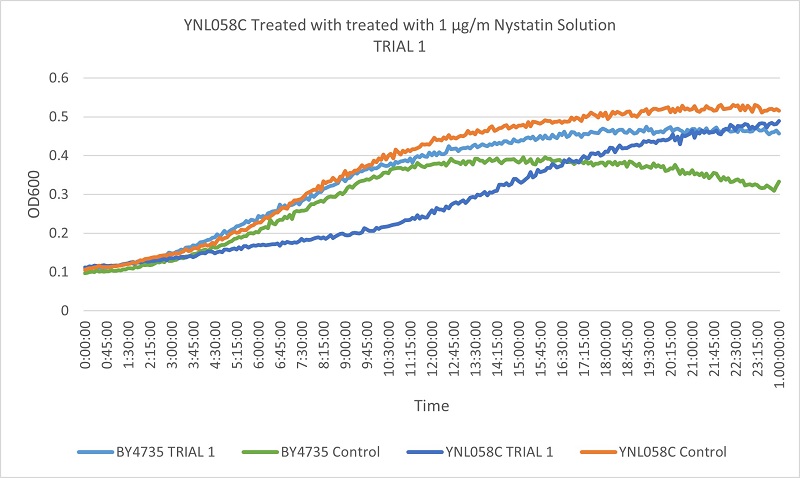
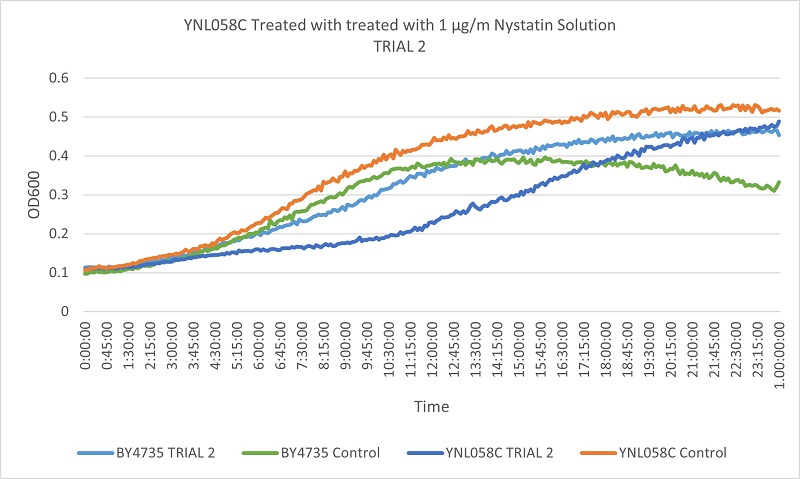
- Knock-out Strain 6:YNL058C
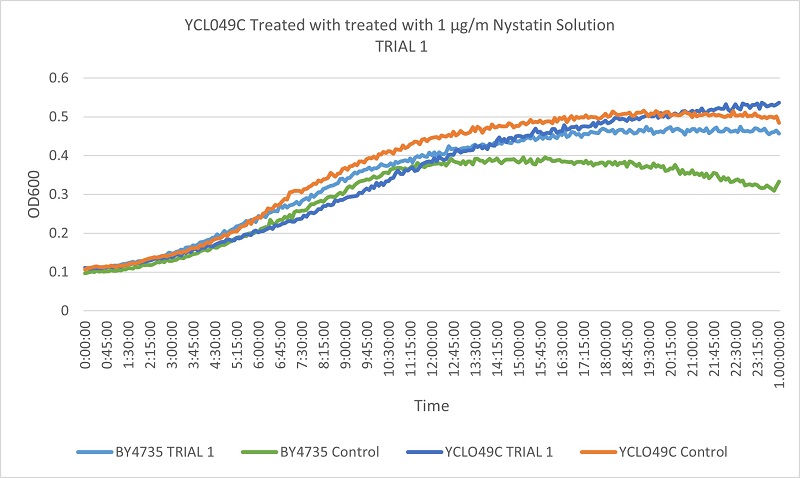
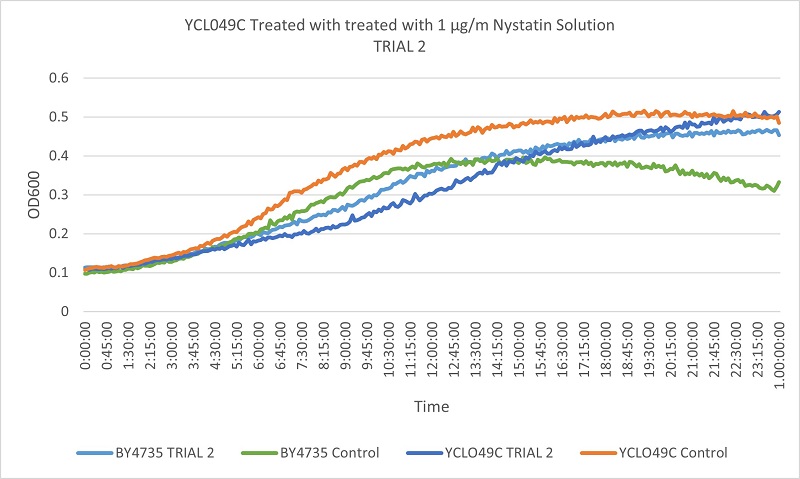
- Knock-out Strain 7:YCL049C
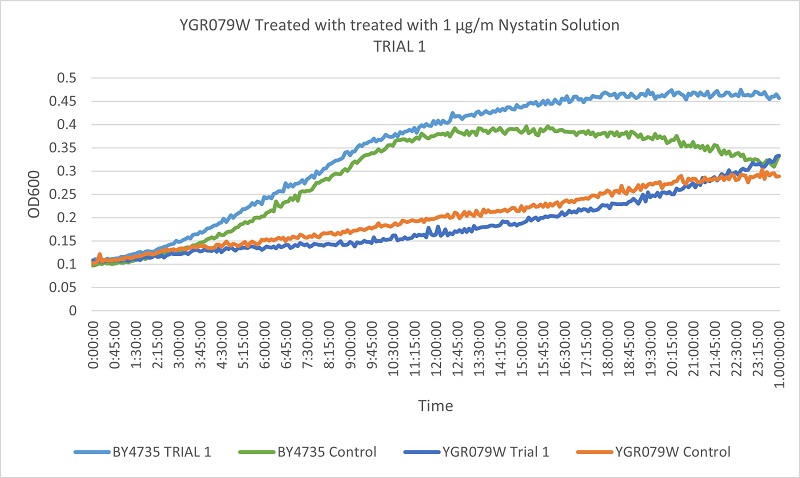
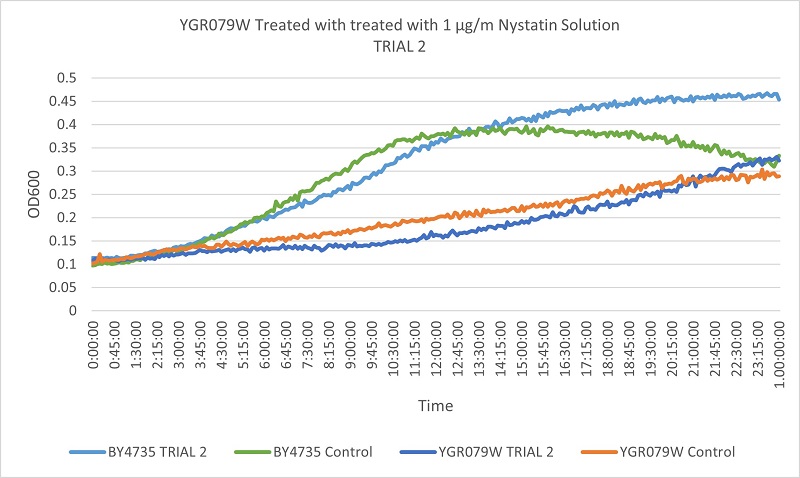
- Knock-out Strain 8:YGR079W
Doubling Times (in Minutes)
- YDL109C
- Trial 1: 12.6
- Trial 2: 12.15789474
- YGL146C
- Trial 1: 12.375
- Trial 2: 12.15789474
- YQR111W
- Trial 1: 12.375
- Trial 2: 12.83333333
- YHL029C
- Trial 1: 11
- Trial 2: 11.74576271
- YDR307W
- Trial 1: 12.375
- Trial 2: 12.83333333
- YNL058C
- Trial 1: 12.15789474
- Trial 2: 12.15789474
- YCL094C
- Trial 1: 11.74576271
- Trial 2: 11.74576271
- YGEO79W
- Trial 1: 18.72972973
- Trial 2: 17.76923077
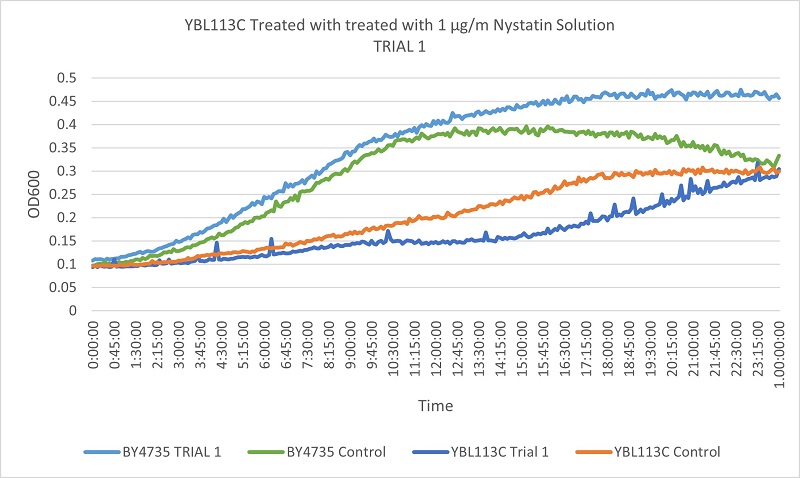
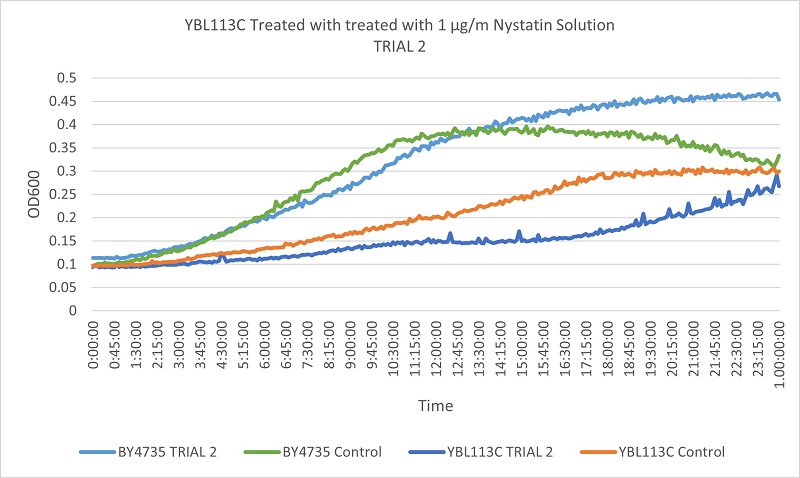
- YBL113C
- Trial 1: 17.76923077
- Trial 2: 20.38235294
Trial one Growth Curves
Trial two Growth Curves