Difference between revisions of "YDL109C"
(→Interpretation) |
(→Interpretation) |
||
| Line 209: | Line 209: | ||
===Interpretation=== | ===Interpretation=== | ||
| − | The wild yeast cell treated with 3% formamide had a doubling time of 876.69 min. The 3% formamide solution added to nine transformed yeast cells was then compared to the wild yeast cell using the computed doubling times to see the effects of the formamide. Where some of the transformed yeast cells were heavily effected by the formamide, the YDL109C strain had doubling times just slightly slower than the wild yeast cell, with a doubling time of 923.43 min. It can be concluded that the transformed yeast cell, YDL109C, | + | The wild yeast cell treated with 3% formamide had a doubling time of 876.69 min. The 3% formamide solution added to nine transformed yeast cells was then compared to the wild yeast cell using the computed doubling times to see the effects of the formamide. Where some of the transformed yeast cells were heavily effected by the formamide, the YDL109C strain had doubling times just slightly slower than the wild yeast cell, with a doubling time of 923.43 min. It can be concluded that the transformed yeast cell, YDL109C, had a slight response to the formamide stress test used in the experiment. |
==References== | ==References== | ||
Revision as of 19:18, 11 May 2022
Share your knowledge...Edit this entry! <protect>
| Systematic name | YDL109C |
| Gene name | |
| Aliases | |
| Feature type | ORF, Uncharacterized |
| Coordinates | Chr IV:267201..265258 |
| Primary SGDID | S000002267 |
Description of YDL109C: Putative lipase; involved in lipid metabolism; YDL109C is not an essential gene[1]
</protect>
Contents
- 1 Community Commentary
- 2 References
Community Commentary
About Community Commentary. Please share your knowledge!
UW-Stout/Heat Shock SP22
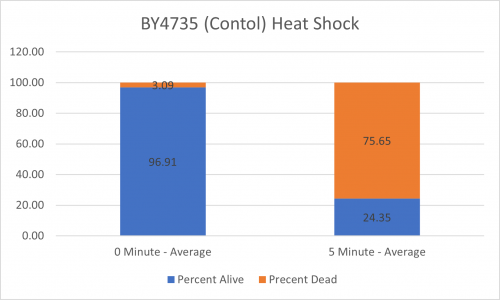
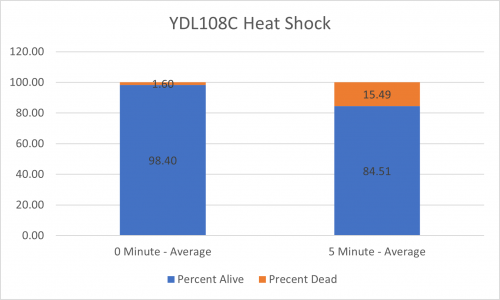
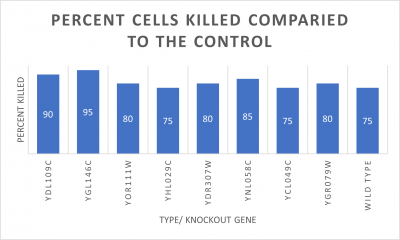
As part of the University of Wisconsin Stout Orphan Gene Project this gene was tested by exposing the cells to heat shock.
Results
Interpretation
From the data gathered, there was a large positive effect to knocking out this gene when it came to the yeast's ability to hold up to heat shock. The modified yeast cells were 347% more resistant to heat shock than the control.
UW Stout/Sucrose Fermentation SP22
| Gene | Glucose | Fructose | Ethanol |
| Standard solution | 2.0000 | 0.2000 | 2.0000 |
| YDl109C | 0.3800 | 0.3933 | 0.3430 |
| YGL140C | 0.2212 | 0.2685 | 0.1867 |
| YOR111W | 0.3332 | 0.3598 | 0.1343 |
| YHL029C | 0.3870 | 0.2368 | 0.1151 |
| YDR307W | 0.4366 | 0.2487 | 0.0606 |
| YNL058C | 0.2710 | 0.3056 | 0.1577 |
| YCL049C | 0.4078 | 0.3052 | 0.1969 |
| YGR079W | 0.4042 | 0.1589 | 0.0080 |
| YBL113C | 0.3498 | 0.2012 | 0.1434 |
| BY4735 | 0.3171 | 0.3084 | 0.3541 |
Interpretation
This gene produced 96.87% of the amount of ethanol that the wild type produced.
UW-Stout/UV Light SP22
As part of the University of Wisconsin Stout Orphan Gene Project this gene was tested under a UV Light using this protocol.
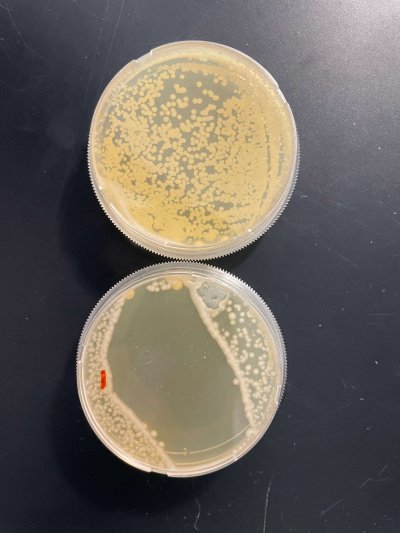
RESULTS
INTERPERTATION
In the graph and photos above you can see that exposing this gene to 600 seconds of 400 Watt UV Light killed approximately 90% of yeast cell cultures, in comparison to its control counterpart which was the the same gene and amount of cells, just was not exposed to UV Light.
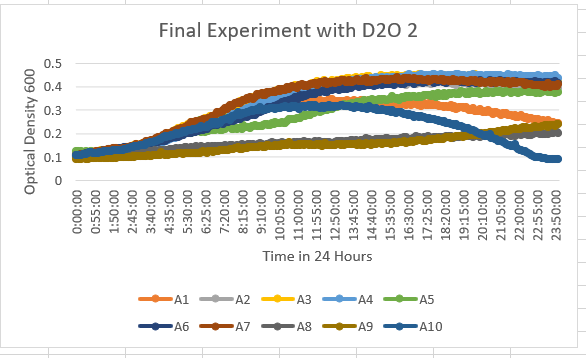
UW-Stout/D2O SP22
The YDL109C (A1) Knock Out Yeast Strain was a little more in the middle spectrum of results with being a little more resistant to the 35% dilution of D2O. The range it stays in as a maximum OD 600 is 0.3-0.4. With the growth curve decaying at the end at about 16 hours.
UW-Stout/Caffeine SP22
As part of the University of Wisconsin Stout Orphan Gene Project this gene was tested by exposing the cells to 4mM of caffeine following this protocol.
Results
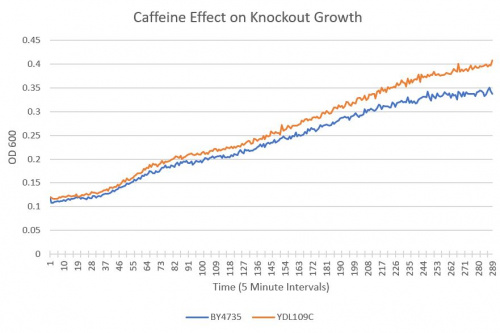
- BY4735(wild type yeast) and YDL109C(knockout yeast gene) growth after being subjected to 4mM caffeine solution.
Interpretation
As can be seen in the growth curve, YDL109C shows a similar response to caffeine as the Wild-Type yeast, however, YDL109C may be slightly more resistant to caffeine.
DNA and RNA Details
Other DNA and RNA Details
Other Topic: expression
Specifically higher expression in phosphorus limited chemostat cultures versus phosphorus excess. [2] [3]
This gene is part of the UW-Stout Orphan Gene Project. Learn more here.
<protect>
UW-Stout/Hydrogen Peroxide SP22
As part of the University of Wisconsin Stout Orphan Gene Project this gene was tested by exposing the cells to hydrogen peroxide.
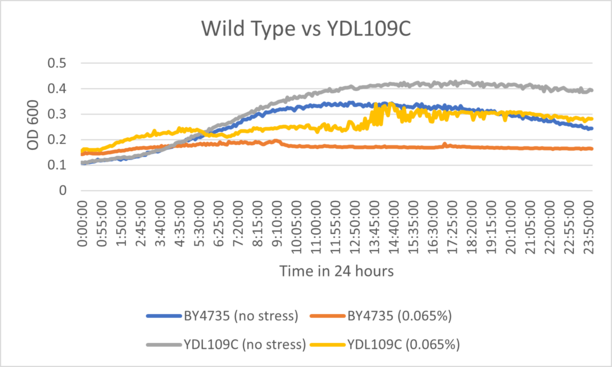
Results
BY4735 and YDL109C after being exposed to 0.065% dilution of hydrogen peroxide solution.
Interpretation
From the growth curve, we can see that YDL109C was inhibited when compared to it's own control, but was still consistent with the rate of growth for unstressed wild type cells. YDL109C may have a higher growth celling the wild strain overall.
UW-Stout/pH(acid) SP22
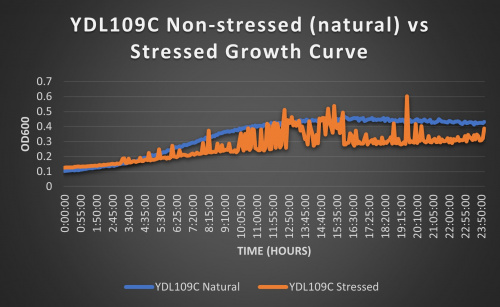 1
Knocked out gene seems to have some effect on cell growth in pH 7 environment based on the flattening of the growth curve. Spikes in stressed line are to be ignored; they occurred due to clumping of cells during analysis. We are focusing on the general linear trend of the growth curve. Please see protocol for specific quantitative doubling time results.
1
Knocked out gene seems to have some effect on cell growth in pH 7 environment based on the flattening of the growth curve. Spikes in stressed line are to be ignored; they occurred due to clumping of cells during analysis. We are focusing on the general linear trend of the growth curve. Please see protocol for specific quantitative doubling time results.
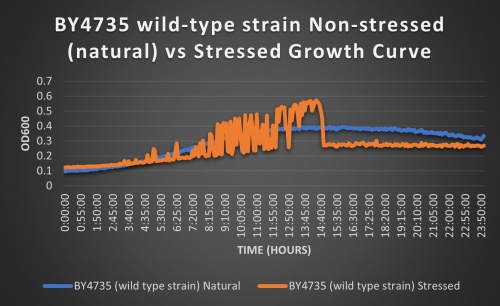 The wild-type strain was used as a control in this experiment; no genes were knocked out, it just represents how the experiment ran on the knocked-out gene strains affects a "typical" cell growth curve. Inclusion of this data on each knock-out strain growth curve graph made for difficult interpretation, so it has been separated. Please refer to this graph as a control when viewing the knock-out strain growth curve graph. Spikes in stressed line are to be ignored; they occurred due to clumping of cells during analysis. We are focusing on the general linear trend of the growth curve.
The wild-type strain was used as a control in this experiment; no genes were knocked out, it just represents how the experiment ran on the knocked-out gene strains affects a "typical" cell growth curve. Inclusion of this data on each knock-out strain growth curve graph made for difficult interpretation, so it has been separated. Please refer to this graph as a control when viewing the knock-out strain growth curve graph. Spikes in stressed line are to be ignored; they occurred due to clumping of cells during analysis. We are focusing on the general linear trend of the growth curve.
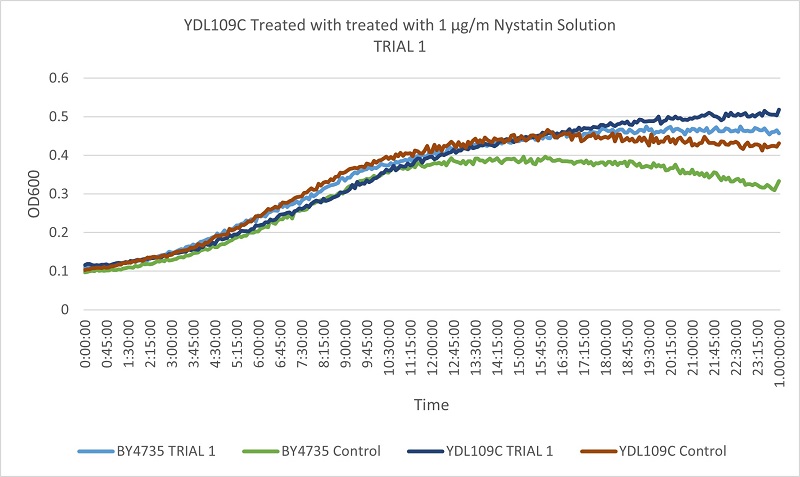
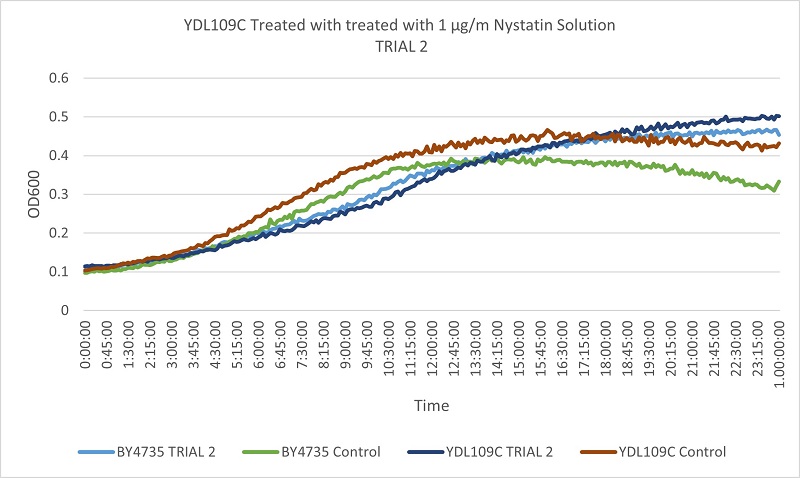
UW Stout/Nystatin SP22
As part of the University of Wisconsin Stout Orphan Gene Project this gene was tested by exposing the cells to 1µg/ml Nystatin solution.
Results
The growth rates of the experimental and control YDL146C strain, when compared to that of the wild type yeast, were similar enough not to indicate a difference in Nystatin tolerance when the YDL146C gene was knocked out.
Results
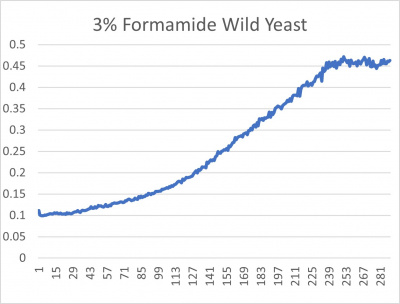
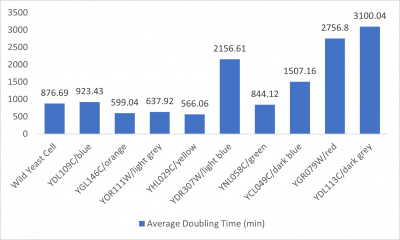
- 3% formamide line graph: x-axis= growing time (min); y-axis= optical density 600
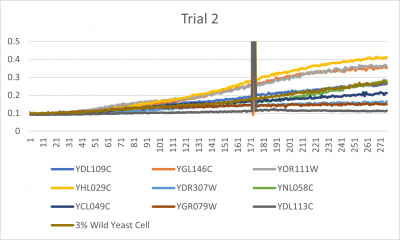
- Trial 2 line graph: x-axis= growing time (min); y-axis= optical density 600; key= yeast strains and corresponding color to the individual lines in the line graph
- Average doubling time bar graph: x-axis= yeast strains and corresponding colors to the trial 2 line graph; y-axis= average doubling time (min)
Interpretation
The wild yeast cell treated with 3% formamide had a doubling time of 876.69 min. The 3% formamide solution added to nine transformed yeast cells was then compared to the wild yeast cell using the computed doubling times to see the effects of the formamide. Where some of the transformed yeast cells were heavily effected by the formamide, the YDL109C strain had doubling times just slightly slower than the wild yeast cell, with a doubling time of 923.43 min. It can be concluded that the transformed yeast cell, YDL109C, had a slight response to the formamide stress test used in the experiment.
References
See Help:References on how to add references
- ↑ Daum G, et al. (1999) Systematic analysis of yeast strains with possible defects in lipid metabolism. Yeast 15(7):601-14 SGD PMID 10341423
- ↑ Boer VM, et al. (2003) The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J Biol Chem 278(5):3265-74 SGD PMID 12414795
- ↑ submitted by Viktor Boer on 2003-07-25
See Help:Categories on how to add the wiki page for this gene to a Category </protect>
UW-Stout/Nonionic Detergent SP22
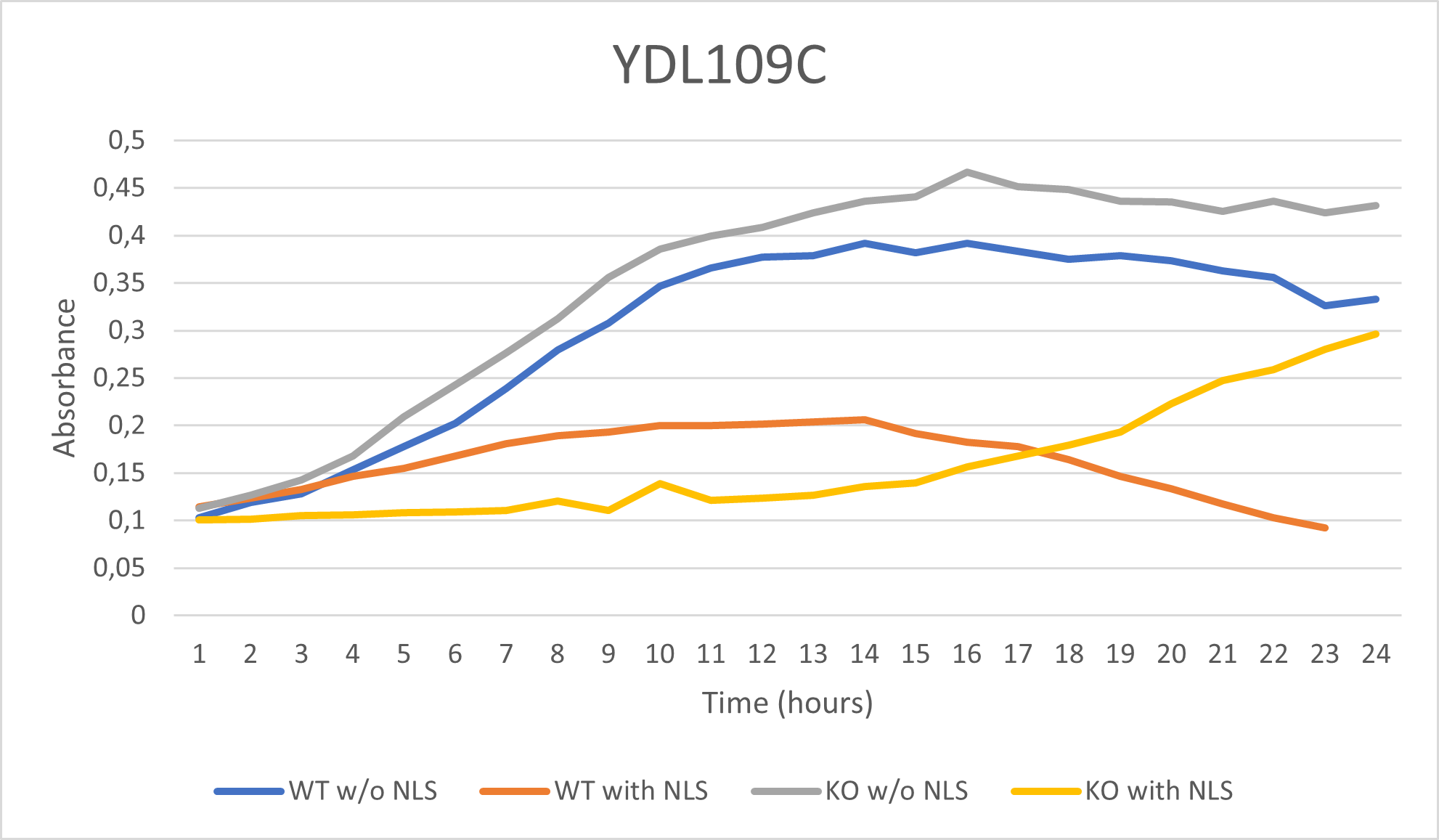
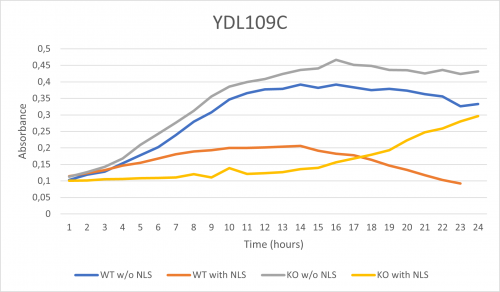
As part of the University of Wisconsin Stout Orphan Gene Project this gene was tested by exposing the cells to 0.001% concentrated NLS. (Nonionic detergent)
Interpretations:
-According to the graph above, we can see that the KO yeast cell YDL109C follow a smaller sensitivity to the stress than the Wild type strain.
-The KO with NLS peaks a lot later than the KO w/o NLS
-We can then conclude that the knocked-out gene in YDL109C doesn't have a significant impact on the growth of the yeast cell.