UW-Stout/Salt Concentration SP21
Contents
Calibration Protocol
Introduction
This protocol will be dependent on results of the highest salt concentration the yeast could grow in without much of a detrimental effect on it. Well 0 will have the wild-type yeast strain and the following wells will have the knock-out strains. All samples will be treated with the same concentration results of the preceding protocol. For this experiment it was determined to be 750 mM of NaCl.
Safety
For this experiment, baseline lab safety requirements are all that’s needed. Eye protection, lab coat, and gloves.
Materials & Equipment
- Salt (NaCl)
- 96-well plate (need 36)
- Pipettes
- Pipette tips
- Yeast Strain (Wild Strain)
- Media
- Tubes
- Sterile Water
- Vortex Machine
- Spectrophotometer
- Incubator set to 30°C
- Basic lab safety equipment (listed in safety)
Protocol
- Vortex the yeast cultures to briefly to resuspend the yeast cells.
- Create 7 different salt solutions. (Calculations listed in section below.)
- Start off by creating a 2M salt solution by adding salt to media. (NaCl = 58.44 g/mol)
- Create 6 more diluted solutions following the values in the NaCl Conc. row listed in the chart below.
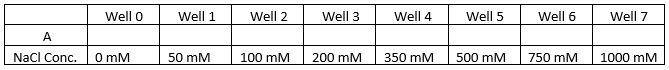
- Set up 8 wells according to the table below.
- To gain a better range this experiment will utilized 24 wells. This is done in order to replicate the experiment three times, expanding the data we would collect.
- In each well there will be 50 ul of the wild strain yeast and media placed prior to adding the 50 ul of different salt concentrations to the wells. Well 0 will host the controlled variable (wild yeast strain with no concentration of salt added) but will add 50 ul of sterile water to that well instead. All wells will have a total volume of 100 ul. Reference Stock Dilution Calculations listed below.
- Set up the plate reader as follows:
- Temperature: 30°C
- Mode: Kinetic
- Wavelength: 600 nm
- Interval: 10 minutes
- Total run time: 12 hours
- Transfer the assay plate to the reader and read for 12 hours.
Stock Dilution Calculations
To keep the added salt concentrations from being diluted in the sterile water, you will be doubling the mM concentration in the calculations to offset that foreseen issue.
- 2000 mM (2M) Stock Solution (Well 7)
- Add 116.88 mg NaCl into 1.5 mL centrifuge tube.
- Add media to the 1.0 mL mark on the tube
- Vortex and then label tube, 2 M
- Calculations for dilutions. Each new mM concentration will take stock from the previously diluted solution, starting from the 2 M master solution and working my way down.
- 1500 mM (Well 6)
- Take 750 uL of 2 M stock solution and place into new tube. Add 250 uL of sterile water to tube, vortex, and then label.
- 1000 mM (Well 5)
- Take 666.7 uL of 1500 mM solution and place into new tube. Add 333.3 uL of sterile water to tube, vortex, and then label.
- 700 mM (Well 4)
- Take 700 uL of 1000 mM solution and place into new tube. Add 300 uL of sterile water to tube, vortex, and then label.
- 400 mM (Well 3)
- Take 571.4 uL of 700 mM solution and place into new tube. Add 428.6 uL of sterile water to tube, vortex, and then label.
- 200 mM (Well 2)
- Take 500 uL of 400 mM solution and place into new tube. Add 500 uL of sterile water to tube, vortex, and then label.
- 100 mM (Well 1)
- Take 500 uL of 200 mM solution and place into new tube. Add 500 uL of sterile water to tube, vortex, and then label.
- 1500 mM (Well 6)
Add 50 uL of each concentration to its respective well location and move onto step 4.
Data & Results
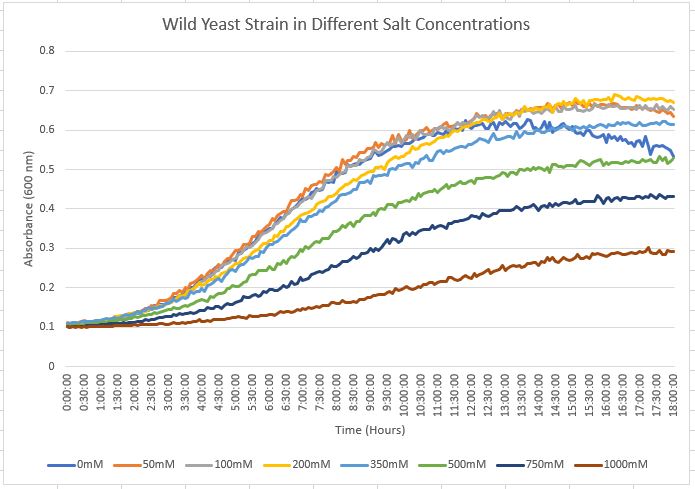
The chart above displays the growth curve for the wild yeast strain against varying concentrations of NaCl. The 750 mM concentration was selected as the wild yeast strain demonstrates a resistance to that concentration and also isn't being overwhelming or too detrimental to the growth rate when compared to the 0 mM data.
Final Protocol
Introduction
This protocol will be dependent on results of the highest salt concentration the yeast could grow in without much of a detrimental effect on it. Well 0 will have the wild-type yeast strain and the following wells will have the knock-out strains. All samples will be treated with the same concentration results of the preceding protocol. Which has been established at 750 mM of NaCl.
Safety
For this experiment, baseline lab safety requirements are all that’s needed. Eye protection, lab coat, and gloves.
Materials & Equipment
- Salt (NaCl)
- 96-well plate (need 36)
- Pipettes
- Pipette tips
- Yeast Strain (Wild Strain & Knockout Strains)
- Media
- Tubes
- Sterile Water
- Vortex Machine
- Spectrophotometer
- Incubator set to 30°C
- Basic lab safety equipment (listed in safety)
Protocol
Procedure:
- Vortex the yeast cultures to briefly to resuspend the yeast cells.
- Create “x” ul of the salt solution decided upon from the previous protocol. This was determined by creating a chart from the previous protocol and observing the concentration that the yeast was most effected by but showing a resistance building up. In the experiment done, the 750 mM concentration was selected. The calculation will follow as 50 ul of salt solution multiplied by the number of strains being tested to get the total amount of salt mixture required.
- Set up “x” wells according to how many strains will be tested. In each well there will be 50 ul of yeast and media placed prior to adding the 50 ul of the decided salt concentration mixed with media to the wells. All wells will have a total volume of 100 ul. In this experiment, 7 wells were utilized. Well 0 for the wild yeast strain and the following six wells for the useable knock out strains the class produced.
- Set up the plate reader as follows:
- Temperature: 30°C
- Mode: Kinetic
- Wavelength: 600 nm
- Interval: 10 minutes
- Total run time: 12 hours
- Transfer the assay plate to the reader and read for 12 hours.
Knockout Strain Data & Results
Click on the following links to see the data and results of the following knockout strains.