UW-Stout/UV Light SP21
Contents
Materials
- Cell 60 mm Culture Dish, containing 6 ml of agar
- Phosphate Buffered Saline (PBS)
- Glycerol stocks of yeast strains
- 15ml centrifuge tubes
Equipment
- Incubator set to 30°C.
- Bachur & Associates Sanata Clara, CA 95050 Model LS-100-3 UV Light Exposeure System
- P1000 and P10 Micro-pipettes
Calibration Protocol
- Wear rubber gloves when handling yeast samples, avoid direct exposure to UV light and wear safety glasses if needed.
- Fill a 15ml centrifuge tube with 9,990ul of PBS.
- Vortex yeast stock to resuspend the yeast cells.
- Pipette 10ul of wild yeast stock into the 9,990ul of PBS to create a dilution containing 2 yeast cells per microliter.
- Vortex dilution and prepare 7 plates with 50ul of the wild yeast dilution for about 100 yeast cells per plate.
- Label each plate individually 0, 500, 600, 700, 800, 900 and 1000 for the number of seconds each plate will be exposed to the UV light.
- Set up the UV light exposure system:
- 400 watts
- desired time increment
- Run yeast plates (without plate top) under the UV light for their respective times in seconds.
- Place plates upside down in dark incubator set to 30°C for 48 hours.
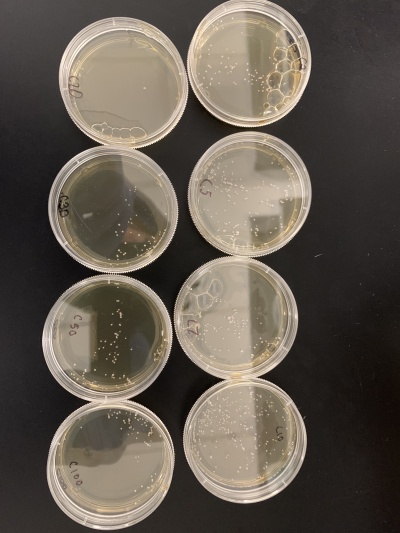
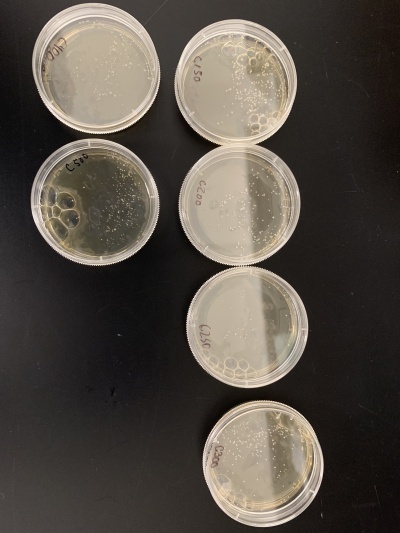
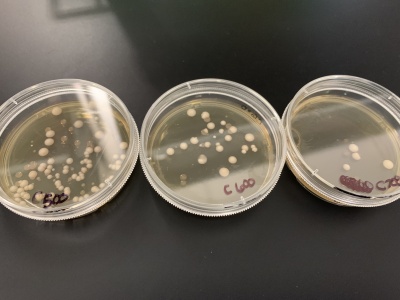
- Count number of colonies on each plate using the 0 second plate as your control to compare to. Based on how many colonies there are on each plate, determine the time frame that killed roughly 50% of the yeast cells.
Calibration Results
- For calibration trial 1 we ran 14 plates at varying times of 2, 5, 7, 10, 20, 30, 50, 100, 150, 200, 250, 300, 400 and 500 seconds. After giving the yeast time to grow
- Results:
Knock-out Protocol
- Wear rubber gloves when handling yeast samples, avoid direct exposure to UV light and wear safety glasses if needed.
- Fill 7 15ml centrifuge tube with 9,990ul of PBS.
- Vortex each yeast stock to resuspend the yeast cells. (wild and 6 knock-out strains)
- Pipette 10ul of each respective yeast stock into one of the centrifuge tubes containing 9,990ul of PBS to create a dilution containing 2 yeast cells per microliter for each strain. Make sure to label each tube so the strains don't get mixed up.
- Vortex each dilution and prepare 14 plates total, two for each strain of yeast with 50ul of the yeast dilution for about 100 yeast cells per plate.
- Label 7 plates with 0 and the other 7 plates with 600 for the number of seconds each plate will be exposed to the UV light. Also label what strain of yeast in in each plate, there should be a 0 plate and 600 plate for each strain.
- Set up the UV light exposure system:
- 400 watts
- desired time increment
- Run yeast plates (without plate top) under the UV light for their respective times in seconds.
- Place plates upside down in dark incubator set to 30°C for 48 hours.
- Count number of colonies on each plate using the 0 second plate as your control to compare to. Based on how many colonies there are on each plate, determine if the knocked-out gene of the yeast had any affect on the survival of the yeast cells.