UW-Stout/Voltage SP23
Pilot Experiment
Materials
- Ice Cold Water
- Ice-Cold Electroporation Buffer (1M D-Sorbitol)
- 0.1M Lithium Acetate
- Room Temperature Electroporation Buffer (1M D-Sorbitol)
- Wild Type Yeast Culture
Equipment
- Electroporator
- Electroporation Cuvettes (Pre-Chilled)
- Centrifuge
- Incubator (30 Degrees Celsius)
- T200 Micropipettor
- Microcentrifuge Tubes
- Biosafety Cabinet
- YPD Plates
Protocol
- Pipette 110 microliters of wild type yeast culture into microcentrifuge tube.
- Wash the cell pellet twice with 200 microliters of ice-cold water at 4500 rpm for 5 min. Discard supernatant.
- Wash the cell pellet once with 200 microliters of ice-cold electroporation buffer (1M D-sorbitol) at 4500 rpm for 5 min. Discard supernatant.
- Wash the cell pellet with 200 microliters 0.1 M Lithium Acetate at 4500 rpm for 5 min. Discard supernatant.
- Wash the cell pellet once with 200 microliters of ice-cold electroporation buffer at 4500 rpm for 5 min. Discard supernatant.
- Suspend the pellet in 200 microliters of electroporation buffer. (Keep cells on ice until electroporation).
- Insert 100 microliters of the mixture into pre-chilled electroporation cuvette. Keep cuvette on ice for 5 minutes before electroporation.
- Electroporate the cells at wanted voltage.
- Insert 100 microliters of YPD into cuvette and place 50 microliters of the mixture on YPD plates to be examined. Place 50 microliters of the non-electroporated mixture onto negative control plates.
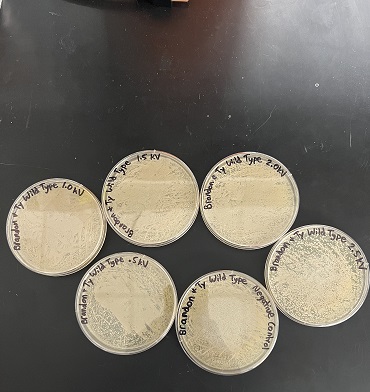
Data
Interpretation
Based on the results of the pilot experiment, we decided on 2.5kV electroporation. This voltage showed the greatest amount of cell stress/death. This was the highest voltage tested.
Final Protocol (Knockout Experiment)
Materials
- Ice Cold Water
- Ice-Cold Electroporation Buffer (1M D-Sorbitol)
- 0.1M Lithium Acetate
- Room Temperature Electroporation Buffer (1M D-Sorbitol)
- Knockout Yeast Strains
Equipment
- Electroporator
- Electroporation Cuvettes (Pre-Chilled)
- Centrifuge
- Incubator (30 Degrees Celsius)
- T200 Micropipettor
- Microcentrifuge Tubes
- Biosafety Cabinet
- YPD Plates
Protocol
- Pipette 110 microliters of each knockout strain into microcentrifuge tubes.
- Wash the cell pellets twice with 200 microliters of ice-cold water at 4500 rpm for 5 min. Discard supernatant.
- Wash the cell pellets once with 200 microliters of ice-cold electroporation buffer (1M D-sorbitol) at 4500 rpm for 5 min. Discard supernatant.
- Wash the cell pellets with 200 microliters 0.1 M Lithium Acetate at 4500 rpm for 5 min. Discard supernatant.
- Wash the cell pellets once with 200 microliters of ice-cold electroporation buffer at 4500 rpm for 5 min. Discard supernatant.
- Suspend the pellets in 200 microliters of electroporation buffer. (Keep cells on ice until electroporation).
- Insert 100 microliters of each mixture into pre-chilled electroporation cuvettes. Keep cuvettes on ice for 5 minutes before electroporation.
- Electroporate the cells at 2.5kV.
- Insert 100 microliters of YPD into each cuvette and place 50 microliters of each mixture on YPD plates to be examined. Place 50 microliters of the non-electroporated mixture onto negative control plates.