Difference between revisions of "UW Stout/Proline FA19"
(→Part 2) |
|||
| Line 41: | Line 41: | ||
#Vortex Solutions | #Vortex Solutions | ||
#Pipet 100ul of each solution in to wells on the microplate | #Pipet 100ul of each solution in to wells on the microplate | ||
| + | |||
| + | |||
| + | |||
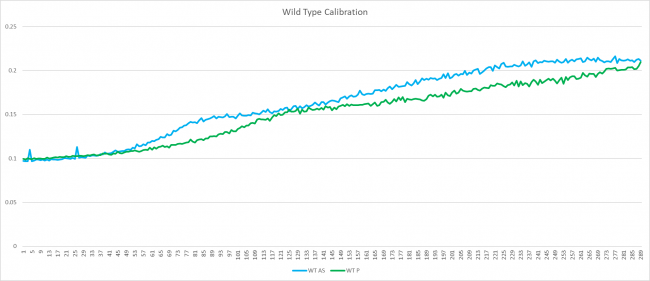
| + | ===Wild Type Calibration Graph=== | ||
| + | [[File:WTCalb.png|650 px]] | ||
Revision as of 17:23, 17 December 2019
Contents
Materials
- Histidine
- Leucine
- uracil
- CSM
- YBN
- Glucose
- Praline
- Ammonium Sulfate
- Water
- Corning COSTAR 96-Well Clear Flat-Bottom Assay Plate
Protocol
Part 1- Making Proline and Ammonium Sulfate Media
- Label two separate 250ml glass media jars with Proline and Ammonium Sulfate
- In the Proline jar mix the following materials
- Histidine 100ml
- Leucine 100ml
- Uracil 100ml
- CSM 0.065g
- YBN 0.171g
- Proline 0.1g
- Fill up to 250ml with water
- In the Ammonium Sulfate jar mix the following materials
- Histidine 100ml
- Leucine 100ml
- Uracil 100ml
- CSM 0.065g
- YBN 0.171g
- Ammonium Sulfate 0.5g
- Fill up to 250ml with water
- Mix well
- Autoclave at 120 degrees Celsius for 15 minutes
- Mix 5ml of glucose to both solutions
Part 2- Adding Yeast to Media
- In biosafety cabinet pipet 1ml of each culture into separate microcentrifuge tubes twice
- Centrifuge tubes of 30 seconds at 5000 RCF
- In biosafety cabinet aspirate supernatant out
- Label tubes with Proline or Ammonium Sulfate
- Add 1ml of either Proline or Ammonium Sulfate
- Vortex Solutions
- Pipet 100ul of each solution in to wells on the microplate