UW Stout/Proline FA19
Contents
Materials
- Histidine
- Leucine
- uracil
- CSM
- YBN
- Glucose
- Praline
- Ammonium Sulfate
- Water
- Yeast wild type and knockout strands
- Corning COSTAR 96-Well Clear Flat-Bottom Assay Plate
Protocol
Part 1- Making Proline and Ammonium Sulfate Media
- Label two separate 250ml glass media jars with Proline and Ammonium Sulfate
- In the Proline jar mix the following materials
- Histidine 100ml
- Leucine 100ml
- Uracil 100ml
- CSM 0.065g
- YBN 0.171g
- Proline 0.1g
- Fill up to 250ml with water
- In the Ammonium Sulfate jar mix the following materials
- Histidine 100ml
- Leucine 100ml
- Uracil 100ml
- CSM 0.065g
- YBN 0.171g
- Ammonium Sulfate 0.5g
- Fill up to 250ml with water
- Mix well
- Autoclave at 120 degrees Celsius for 15 minutes
- Mix 5ml of glucose to both solutions
Part 2- Adding Yeast to Media
- In biosafety cabinet pipet 1ml of each culture into separate microcentrifuge tubes twice
- Centrifuge tubes of 30 seconds at 5000 RCF
- In biosafety cabinet aspirate supernatant out
- Label tubes with Proline or Ammonium Sulfate
- Add 1ml of either Proline or Ammonium Sulfate
- Vortex Solutions
- Pipet 100ul of each solution in to wells on the microplate
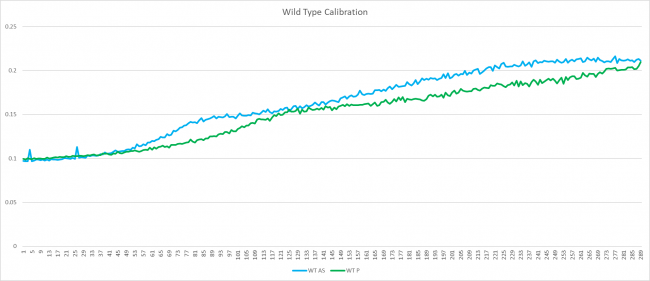
Wild Type Calibration Graph
In the graph above, the wild type that grew in ammonium sulfate media grew faster than in proline media. The doubling rate in proline was 113.99 minutes and in the ammonium sulfate media had a doubling rate of 78.6 minutes and therefore the wild type grows slower in proline media compared to ammonium sulfate media.