UW-Stout/Caffeine SP23
Knockout protocols
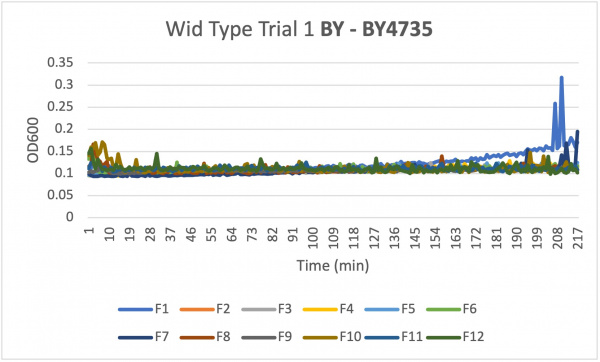
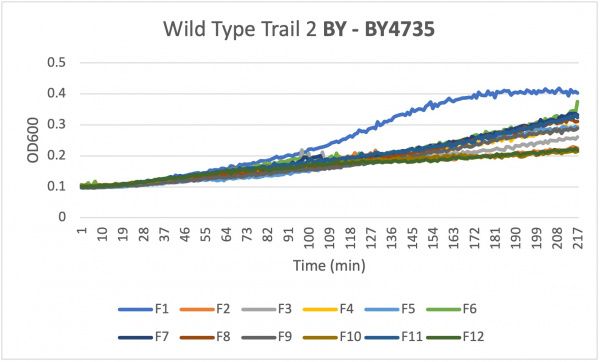
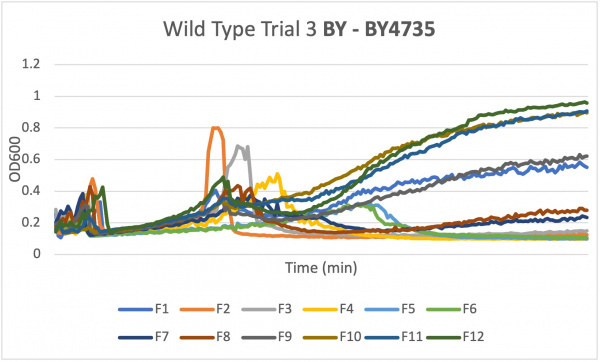
- Caffeine is a purine that affects cellular processes. Effects of yeast cells growth sensitivity to caffeine are often associated with defects in cell signaling pathways. During the Spring of 2023 the Intro Cell and Molecular Biology class created their own experiments to see how the three final knocked out yeast strains would live under certain conditions. This page is about using caffeine to see how the certain yeast strains live.
Materials
- Wild Type Yeast Gene (50ul per well)
- Knockout Strains
- Caffeine Powder (given to us by instructor)
- Microwell Plate (new one for each day)
- 12 microcentrifuge tubes
- Sterile Water
Equipment
- Sterile Hood Environment
Protocol
Dilution
- First compute the dilutions in the stock solution for the max amount of caffeine and water dilution
- Comes to a total of 10mL of sterile water and 0.00194 mg of caffeine powder
- Then measure out the correct amount of mg of caffeine in a mg weigh scale
- Measure out 10mL of sterile water in a tube
- 10mL=1000ul
- Once water is measured out then add the caffeine powder to the tube
- Mix until all particles are dissolved
- BREAK
- While particles are dissolving compute the dilutions for twelve tubes
Microliters of Caffeine Concentrate Microliters of H20
- Then take 12 microcentrifuge tubes and label them 1-12
- Once particles are all dissolved then started adding the correct amount of caffeine concentrate and the H20 to the tubes
- Once completed vortex the tubes to ensure it is fully mixed
- Then store in a microcentrifuge tube rack at room temperature
Adding Yeast Wild Type
- Once all dilutions are complete then you can move onto the next step
- Spray off tubes and rack with ethanol to ensure they are sterile before going into the sterile environment
- Make sure all steps are being completed in the sterile hood
- Find and pick a well letter and start to load the wells
- Make your first well as your control by adding 50ul of water and 50ul of the wild type yeast cells
- The following wells should contain 50ul of your treatment and 50ul of the wild type yeast cells
- Once completed make sure to close the hood
- Once everyone has completed this step the instructor will take the plate from here
- Once your data has been collected three times and the data is similar you can move onto the knockout strains
Adding Yeast Knockout Strains
- Spray off tubes and rack with ethanol to ensure they are sterile before going into the sterile environment
- Make sure all steps are being completed in the sterile hood
- Find and pick a well letter and start loading the wells
- Vortex yeast cells briefly
- You will want to pick your three dilutions that grew the most with the wild type yeast
- The first well will be your control with 50ul of water and 50ul of the knockout strain 1
- The three remaining wells will contain your treatment with the knockout strain 1
- You will repeat this process two more times with the other knockout strains
- Close the hood when finished
- Set up plate reader
- Temperautre 30 degrees C
- Mode Kinetic
- Wavelenght 600nm
- Interval 5 min
- Total time run 24 hours
- Shake before read 30 seconds
- Transfer plate and read for 24 hours