YOR387C
Share your knowledge...Edit this entry! <protect>
| Systematic name | YOR387C |
| Gene name | |
| Aliases | |
| Feature type | ORF, Uncharacterized |
| Coordinates | Chr XV:1070241..1069621 |
| Primary SGDID | S000005914 |
Description of YOR387C: Putative protein of unknown function; regulated by the metal-responsive Aft1p transcription factor; highly inducible in zinc-depleted conditions; localizes to the soluble fraction[1][2][3]
</protect>
Contents
Community Commentary
About Community Commentary. Please share your knowledge!
This gene is part of the UW-Stout Orphan Gene Project. Learn more here.
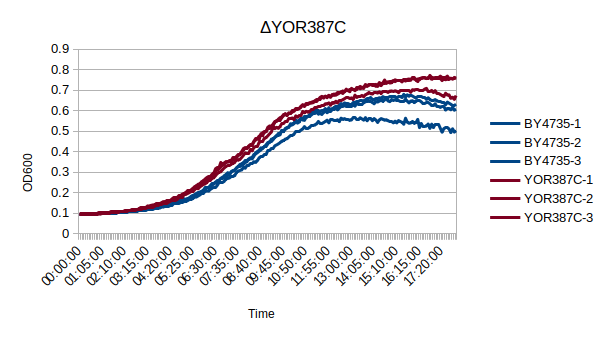
Growth Curve
In a BY4735 background, knocking out YOR387C does not seem to have much effect on the strain's growth rate. In this experiment, the BY4735 strain's doubling time was 162 minutes, while the YOR387C knock-out strain's doubling time was 137 minutes. (These doubling times are the means of three experiments.)
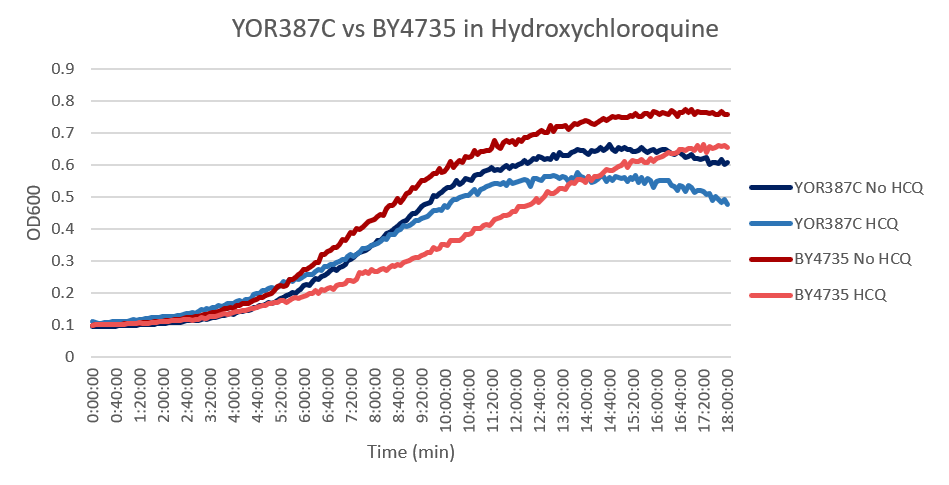
Hydroxychloroquine
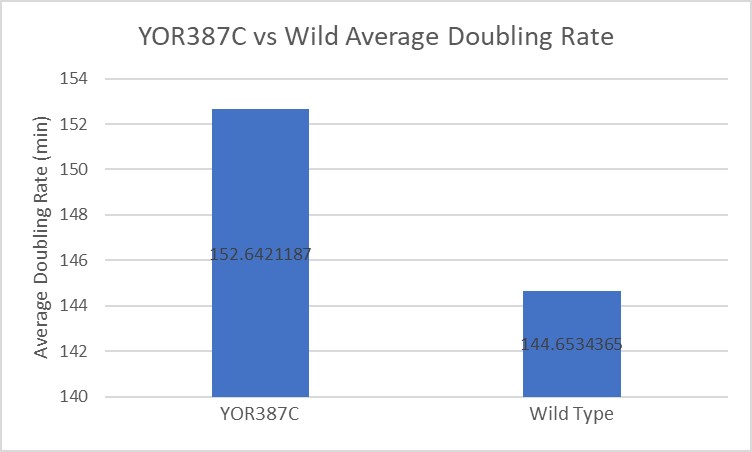
Under normal conditions, the BY4753 doubling time is 144 minutes, while that of YOR387C is 158 minutes. On average, the YOR387C lag phase was slightly longer than that of BY4753 under normal conditions. When grown in an environment with hydroxychloroquine, YOR387C doubled in 249 minutes, while BY4753 doubled in 276 minutes. This shows that hydroxychloroquine inhibits BY4753 slightly more than YOR387C. (These doubling times and curves are the means of three experiments.)
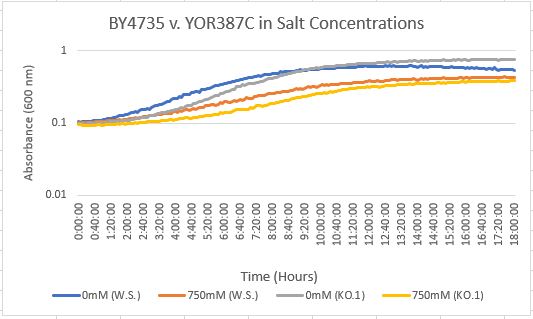
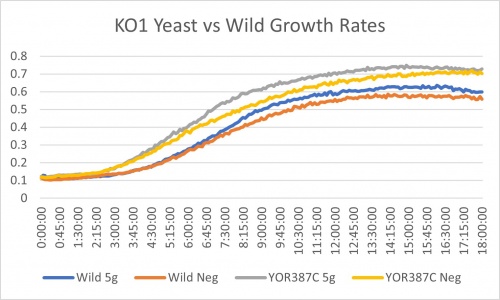
Salt Concentration (NaCl)
0mM NaCl conc. BY4735 strain's doubling time: 169 minutes
0mM NaCl conc. YOR387C strain's doubling time: 129 minutes
750mM NaCl conc. BY4735 strain's doubling time: 294 minutes
750mM NaCl conc. YOR387C strain's doubling time: 282 minutes
The graph above shows the growth rate for the previously listed strains and the relative level of NaCl concentration. Knocking out the gene positively influences the growth rate. Comparing the YOR387C doubling times in 0mM and 750mM NaCl, there is a large effect on the growth rate. This effect negatively impacts the knockout strain's (YOR387C) growth rate.
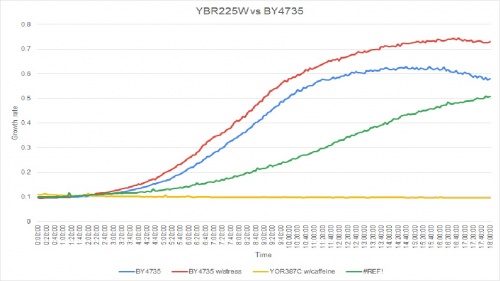
Caffeine Group 1
Caffeine had very detrimental effect on the YOR387C strain; it had completely blocked the yeast's growth rate. The caffeine effected strain had not grown. The doubling times are as follows:
Doubling time of YOR387C with caffeine: 458 minutes
Doubling time of YOR387C without caffeine: 122 minutes
Doubling time of BY4735 with caffeine: 138 minutes
Doubling time of BY4735 without caffeine: 187 minutes
Competative Co-culture
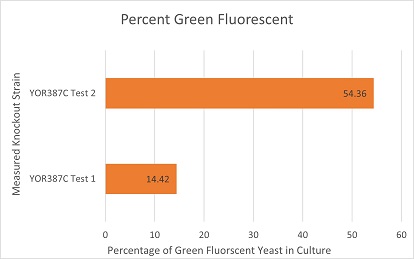
These are the results of a competitive co-culture protocol. The knockout strain was grown in a culture to test fitness against a green, fluorescent wild-type strain. The percentage of analyzed cells in the culture measured was those displaying green fluorescence. In theory, this should mean if a knockout has reduced the fitness of a strain of yeast, the fluorescent wild-type strain would have a higher percentage than the knockout strain. If the knockout does not decrease fitness, they would be roughly equal. This may not be so in results. Sources of error may include contamination and human error.
The results here are not expected in theory. Test 1 was more likely than not contaminated with a faster growing organism than yeast that did not possess fluorescence. Strain 2 gave an expected result of about a 1:1 ratio that suggests the knockout of YOR378C in yeast does not reduced its fitness in a fair, competitive scenario.
Ammonium Sulfate
YOR387C gene does not show any major sensitivities to nitrogen starvation. The growth rate is 7.3% slower in the negative control than that of the 5g/ml treatment. The wild strains comparative growth rate was 17.8% slower. This is not much different in the scope of the data, but it could indicate that YOR387C is slightly less sensitive to nitrogen fixation than the wild strain.
<protect>
UV Light
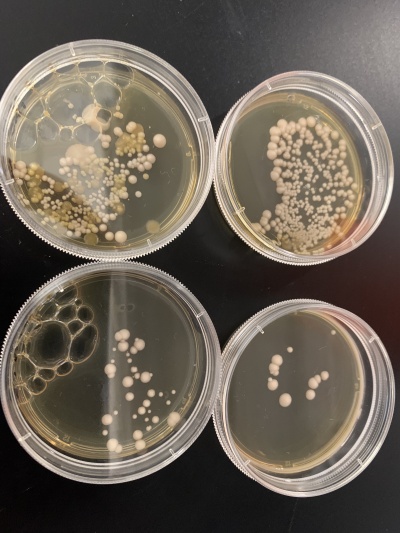
Results:
- Experiment 1:0sec=342 colonies, 600sec=13 colonies.
- Experiment 2:0sec=222 colonies, 600sec=42 colonies.
Interpretation: The two 0sec plates are on top and the 600sec plates are on the bottom. Assuming the plates had around the same amount of yeast cells, the UV light killed a majority of the yeast cells meaning it's possible that the knocked-out gene had something to do with UV resistance/DNA repair.
Calcofluor White
From this data we can determine that there was a moderate growth inhibition on this strain of yeast. The modified yeast had a doubling rate that was eight minutes longer than the wild type on average, and when compared to the effect on other yeast strains this is a small difference, but not insignificant.
Heat Shock
The YOR387C yeast gene was effected greatly by the heat shocking from the thermal cycler. As can be seen in the visual, the controlled plate (non-heat shocked) essentially grew a thick blanket of very tiny DNA colonies throughout the entire plate. Both trials of the actual heat shocked plates resembled roughly the same outcome. Both grew very small amounts of medium-sized colonies. This basically means that this strain was fragile and the heat killed off most of the potential colonies while being grown.
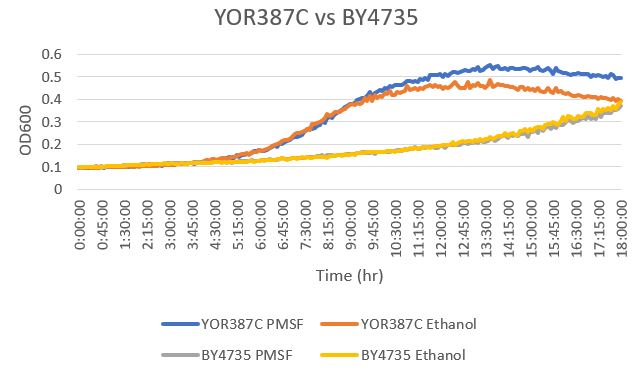
UW-Stout/Phenylmethylsulphonyl Fluoride SP21|Phenylmethylsulphonyl Fluoride
References
See Help:References on how to add references
- ↑ Higgins VJ, et al. (2003) Application of genome-wide expression analysis to identify molecular markers useful in monitoring industrial fermentations. Appl Environ Microbiol 69(12):7535-40 SGD PMID 14660410
- ↑ Rutherford JC, et al. (2003) Aft1p and Aft2p mediate iron-responsive gene expression in yeast through related promoter elements. J Biol Chem 278(30):27636-43 SGD PMID 12756250
- ↑ Terashima H, et al. (2002) Sequence-based approach for identification of cell wall proteins in Saccharomyces cerevisiae. Curr Genet 40(5):311-6 SGD PMID 11935221
See Help:Categories on how to add the wiki page for this gene to a Category </protect>