Difference between revisions of "YLR006C"
(→Bases) |
(→Caffeine) |
||
| (59 intermediate revisions by the same user not shown) | |||
| Line 29: | Line 29: | ||
==Community Commentary== | ==Community Commentary== | ||
{{CommentaryHelp}} | {{CommentaryHelp}} | ||
| + | |||
| + | |||
=== Protein Details === | === Protein Details === | ||
[[Category:Topic:Protein Details]] | [[Category:Topic:Protein Details]] | ||
| Line 37: | Line 39: | ||
Identified as an efficient substrate of Clb2-Cdk1-as1 in a screen of a proteomic GST-fusion library. <ref name='S000074306'>Ubersax JA, et al. (2003) Targets of the cyclin-dependent kinase Cdk1. Nature 425(6960):859-64 {{SGDpaper|S000074306}} PMID 14574415</ref> <ref name = 'CAset7903-2004-01-29'>submitted by [http://db.yeastgenome.org/cgi-bin/colleague/colleagueSearch?id=7903 Jeff Ubersax] on 2004-01-29</ref> | Identified as an efficient substrate of Clb2-Cdk1-as1 in a screen of a proteomic GST-fusion library. <ref name='S000074306'>Ubersax JA, et al. (2003) Targets of the cyclin-dependent kinase Cdk1. Nature 425(6960):859-64 {{SGDpaper|S000074306}} PMID 14574415</ref> <ref name = 'CAset7903-2004-01-29'>submitted by [http://db.yeastgenome.org/cgi-bin/colleague/colleagueSearch?id=7903 Jeff Ubersax] on 2004-01-29</ref> | ||
| − | {{ShortCenteredHR}} | + | ==Acids== |
| + | [[UW-Stout/Acids_SP23]] | ||
| + | |||
| + | [[File:MEL_control_v.s_knockout-2.jpg]] | ||
| + | |||
| + | ===Analysis=== | ||
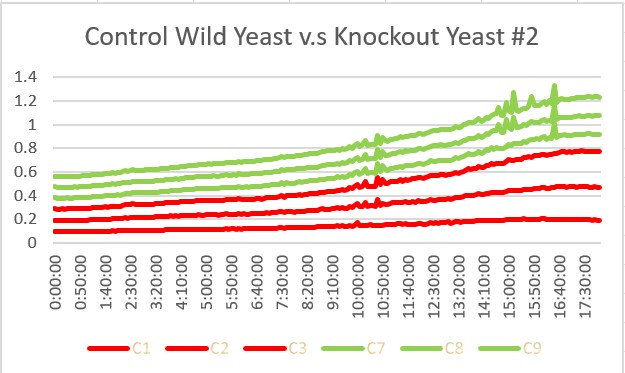
| + | These are the results we gathered from testing a pH of # against the control wild yeast and the gene YKL162C. The three red lines are the growth rates of the BY (wild yeast) mixed with a pH of # and the three green lines are the three growth rates of the SSK1 knockout gene mixed with a pH of #. All trials showed strong cell growth. The SSK1 knockout gene had an average doubling time of 22.7 minutes and the wild yeast had an average doubling time of 13.57 minutes. Overall, the SSK1 gene was able to have more rapid cell growth over the 18 hour period. | ||
| + | |||
| + | ==Caffeine== | ||
| + | [[UW-Stout/Caffeine SP23]] | ||
| + | |||
| + | [[File:KnockoutGeneControl.jpg|center|thumb|600px]] | ||
| + | |||
| + | [[File:KnockoutGene2.jpg|center|thumb|600px]] | ||
| + | |||
| + | '''Analysis''' | ||
| + | |||
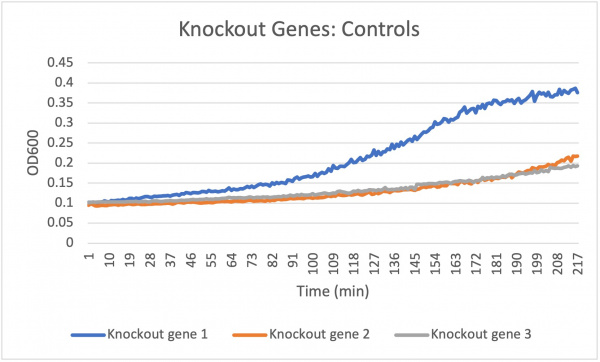
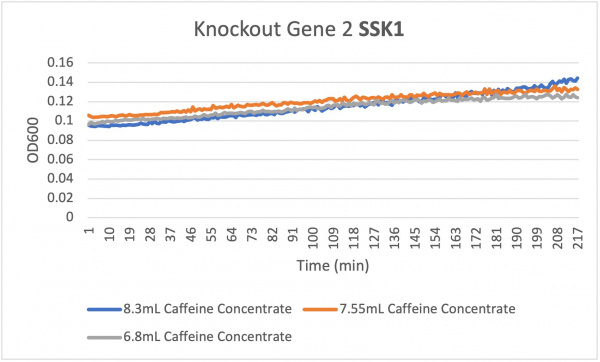
| + | The average doubling time for the knockout gene SSK1 under standard conditions was 60 minutes. Under the stress of the caffeine the doubling time decreased to 24 minutes. Under the stress of caffeine we see growth happening showing that the amount of caffeine in the concentration was low enough in order to produce growth. Based off of the different concentration levels we notice that they are increasing in growth rate very evenly. The 7.55 and 6.8 mL concentrations remain constant around 208 min while the 8.3 mL concentration increases slightly in growth. This shows that the higher concentration of caffeine under the knockout gene SSK1 had a slightly faster growth rate compared to the other concentrations 7.55 and 6.8. | ||
| + | |||
| + | {{ShortCenteredHR}} | ||
==Glucose Sensitivity== | ==Glucose Sensitivity== | ||
| − | [https://wiki.yeastgenome.org/index.php/UW-Stout/Glucose_SP23] | + | |
| + | ====Protocol==== | ||
| + | [https://wiki.yeastgenome.org/index.php/UW-Stout/Glucose_SP23] | ||
| Line 47: | Line 70: | ||
===Analysis=== | ===Analysis=== | ||
| − | The | + | |
| + | |||
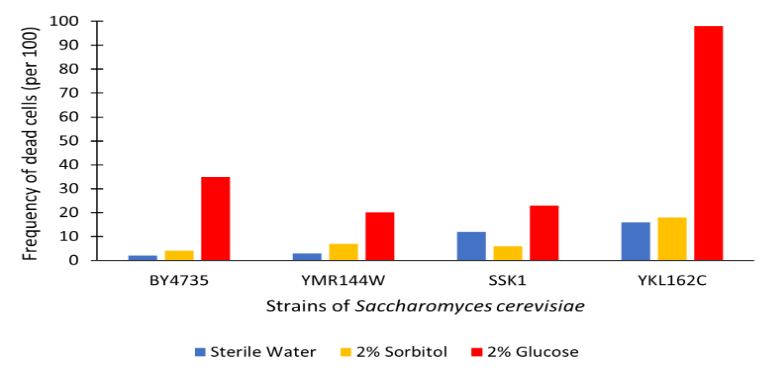
| + | The graph displayed above shows a viability assay of SSK1 knockout gene as compared to BY4735 (wild type) and other knockout strains. We found that there was about 20% less cell death then in the control wild type. It was surprising to find that by removing this gene there was an increase in cell viability. Further research may be conducted to test this slight resistance to all cell death, but more importantly the resistance to glucose-induced apoptosis as noted in the wild type BY4735 for more conclusive results. | ||
==Isopropanol== | ==Isopropanol== | ||
| Line 110: | Line 135: | ||
==Bases== | ==Bases== | ||
| − | [[Image:EMYeast2 | + | [[Image:EMYeast2.jpg]] |
| + | |||
| + | '''Analysis''' Yeast 2, A4 A5 A6, showed almost no growth compared to the Wild Type yeast, A1 A2 A3. This knockout gene likely did not benefit the resistance to basic pH changes in the environment. | ||
| + | |||
| + | Protocol- | ||
[[UW-Stout/Bases SP23]] | [[UW-Stout/Bases SP23]] | ||
| + | |||
| + | |||
| + | == Electrical Shock == | ||
| + | |||
| + | '''Protocol''' | ||
| + | |||
| + | [[UW-Stout/Voltage SP23]] | ||
| + | |||
| + | '''Data''' | ||
| + | |||
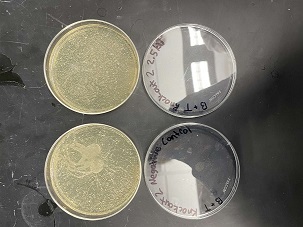
| + | [[Image:21.jpg]] | ||
| + | |||
| + | [[Image:22.jpg]] | ||
| + | |||
| + | '''Analysis''' | ||
| + | |||
| + | These two pictures are showing how the second knockout strain reacts with either no electrical shock or an electrical shock of 2.5 kV. These were the same experiments but on different days to make sure things looked like each other. With our second knockout we notice something very unusual. When we shock this strain with 2.5kV the cells seem to not die at all, as if they are resistant to the shock. This happened with both trials. With our second trial, we noticed that we had contamination in all of our plates. While our cells did grow so did other things. | ||
==References== | ==References== | ||
Latest revision as of 18:37, 9 May 2023
Share your knowledge...Edit this entry! <protect>
| Systematic name | YLR006C |
| Gene name | SSK1 |
| Aliases | |
| Feature type | ORF, Verified |
| Coordinates | Chr XII:163893..161755 |
| Primary SGDID | S000003996 |
Description of YLR006C: Cytoplasmic response regulator; part of a two-component signal transducer that mediates osmosensing via a phosphorelay mechanism; required for mitophagy; dephosphorylated form is degraded by the ubiquitin-proteasome system; potential Cdc28p substrate[1][2][3][4]
</protect>
Contents
Community Commentary
About Community Commentary. Please share your knowledge!
Protein Details
Protein Modification
Modification(s): Phosphorylation
Identified as an efficient substrate of Clb2-Cdk1-as1 in a screen of a proteomic GST-fusion library. [4] [5]
Acids
Analysis
These are the results we gathered from testing a pH of # against the control wild yeast and the gene YKL162C. The three red lines are the growth rates of the BY (wild yeast) mixed with a pH of # and the three green lines are the three growth rates of the SSK1 knockout gene mixed with a pH of #. All trials showed strong cell growth. The SSK1 knockout gene had an average doubling time of 22.7 minutes and the wild yeast had an average doubling time of 13.57 minutes. Overall, the SSK1 gene was able to have more rapid cell growth over the 18 hour period.
Caffeine
Analysis
The average doubling time for the knockout gene SSK1 under standard conditions was 60 minutes. Under the stress of the caffeine the doubling time decreased to 24 minutes. Under the stress of caffeine we see growth happening showing that the amount of caffeine in the concentration was low enough in order to produce growth. Based off of the different concentration levels we notice that they are increasing in growth rate very evenly. The 7.55 and 6.8 mL concentrations remain constant around 208 min while the 8.3 mL concentration increases slightly in growth. This shows that the higher concentration of caffeine under the knockout gene SSK1 had a slightly faster growth rate compared to the other concentrations 7.55 and 6.8.
Glucose Sensitivity
Protocol
Analysis
The graph displayed above shows a viability assay of SSK1 knockout gene as compared to BY4735 (wild type) and other knockout strains. We found that there was about 20% less cell death then in the control wild type. It was surprising to find that by removing this gene there was an increase in cell viability. Further research may be conducted to test this slight resistance to all cell death, but more importantly the resistance to glucose-induced apoptosis as noted in the wild type BY4735 for more conclusive results.
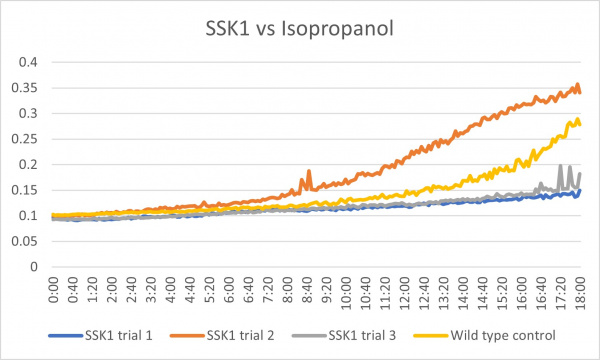
Isopropanol
Analysis
These results were what was expected with only a little bit of difference. The first trial and third trials were close and there was less growth than the control which could have been because the knockout genes were more sensitive to the isopropanol. The second trial gave different results in which there was more growth than the control which is the opposite of the first and third. This could have been caused by an error because there was consistency in the other two.
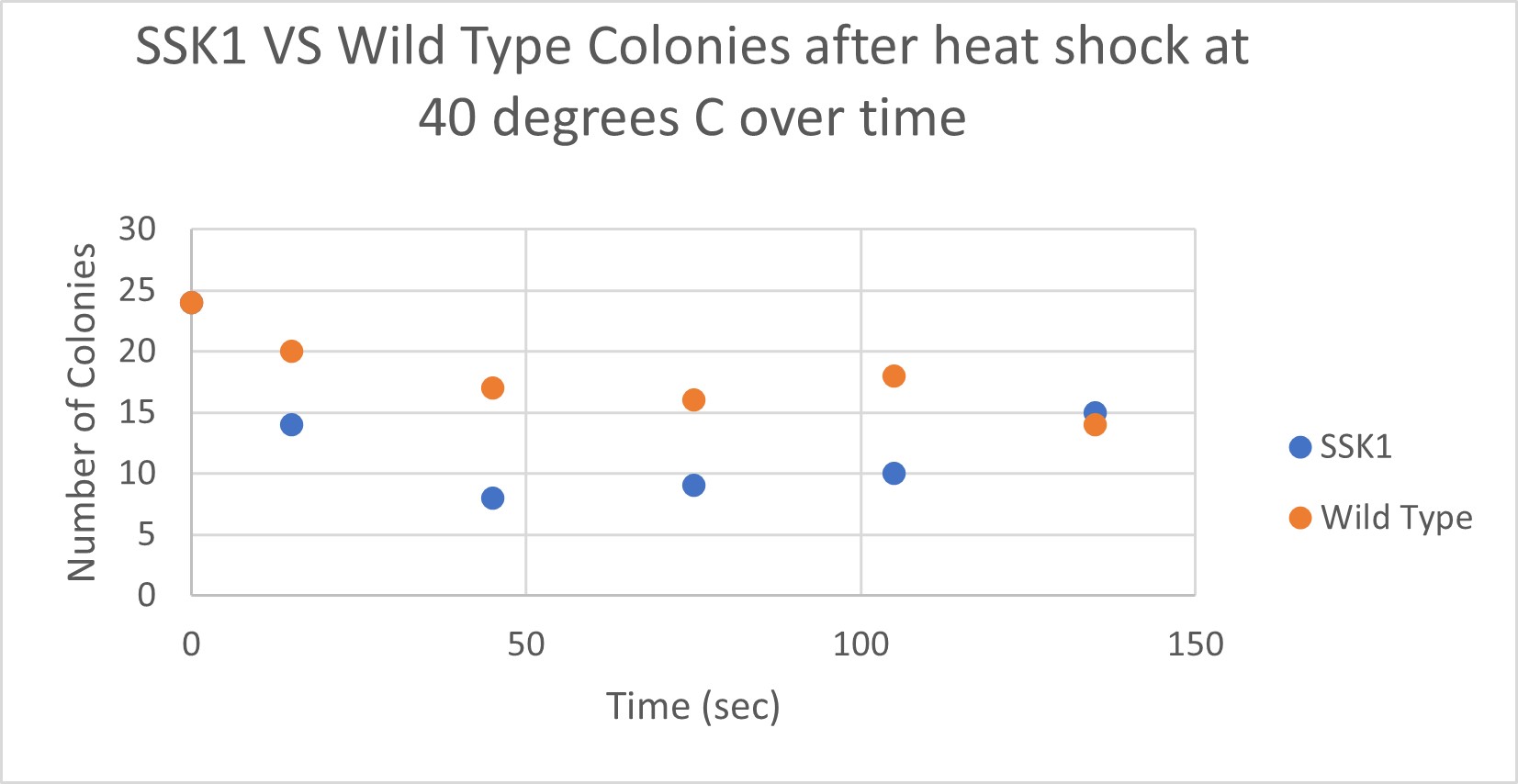
Heat Shock
| Time (sec) | Colonies | |
|---|---|---|
| 0 | 24 | |
| 15 | 14 | |
| 45 | 8 | |
| 75 | 9 | |
| 105 | 10 | |
| 135 | 15 |
| Time (sec) | Colonies | |
|---|---|---|
| 0 | 24 | |
| 15 | 20 | |
| 45 | 17 | |
| 75 | 16 | |
| 105 | 18 | |
| 135 | 14 |
Analysis
The general trend of our data yielded a negative relationship between time of heat shock. About half of the yeast cells died off in a matter of 135 seconds at 40 degrees Celsius. The SSK1 yeast were dying off at a very comparable rate over time compared to the Wild Yeast strain. This means that the gene that was knocked out must not have a correlation with heat shock.
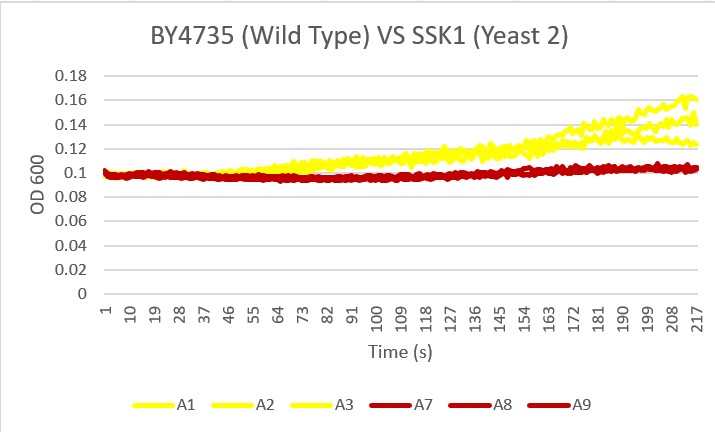
Bases
Analysis Yeast 2, A4 A5 A6, showed almost no growth compared to the Wild Type yeast, A1 A2 A3. This knockout gene likely did not benefit the resistance to basic pH changes in the environment.
Protocol- UW-Stout/Bases SP23
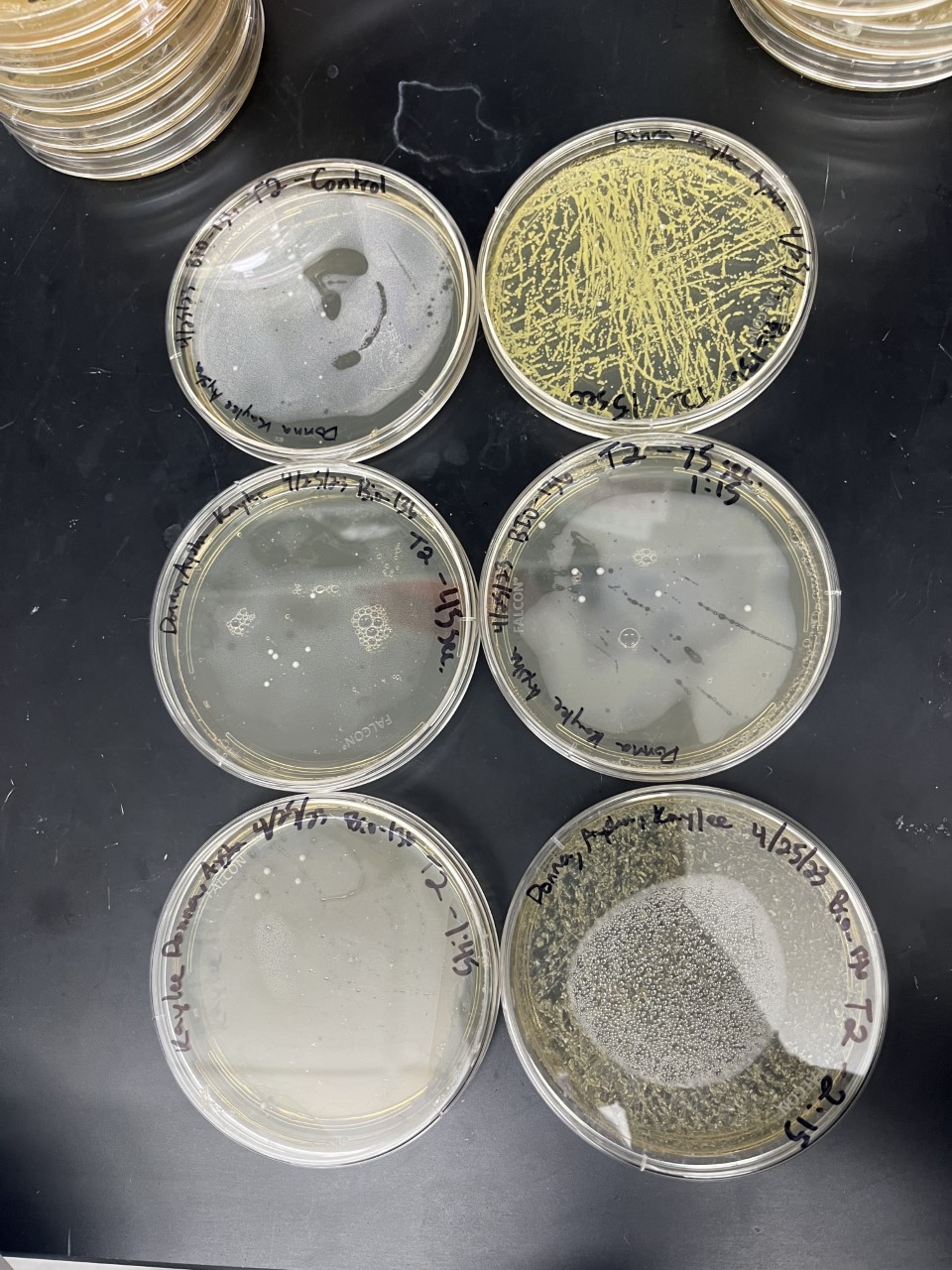
Electrical Shock
Protocol
Data
Analysis
These two pictures are showing how the second knockout strain reacts with either no electrical shock or an electrical shock of 2.5 kV. These were the same experiments but on different days to make sure things looked like each other. With our second knockout we notice something very unusual. When we shock this strain with 2.5kV the cells seem to not die at all, as if they are resistant to the shock. This happened with both trials. With our second trial, we noticed that we had contamination in all of our plates. While our cells did grow so did other things.
References
See Help:References on how to add references
- ↑ Mao K, et al. (2011) Two MAPK-signaling pathways are required for mitophagy in Saccharomyces cerevisiae. J Cell Biol 193(4):755-67 SGD PMID 21576396
- ↑ Posas F, et al. (1996) Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 two-component osmosensor. Cell 86(6):865-75 SGD PMID 8808622
- ↑ Sato N, et al. (2003) Phosphorelay-regulated degradation of the yeast Ssk1p response regulator by the ubiquitin-proteasome system. Mol Cell Biol 23(18):6662-71 SGD PMID 12944490
- ↑ 4.0 4.1 Ubersax JA, et al. (2003) Targets of the cyclin-dependent kinase Cdk1. Nature 425(6960):859-64
SGD PMID 14574415 Cite error: Invalid
<ref>tag; name "S000074306" defined multiple times with different content - ↑ submitted by Jeff Ubersax on 2004-01-29
See Help:Categories on how to add the wiki page for this gene to a Category </protect>