UW-Stout/Fermentation
Protocol: Fermentation of Yeast
Materials
• 2 1-liter PYREX Bottle
• Sunrise Science fermentation media kit
• 5 g Ammonium Sulfate
• .5 L 50% w/v sucrose solution
• 10 ml of Histodine (200mg/100ml), 10 ml of Uracil(200mg/100ml), 10 ml of Leucine (1.2g/100ml)
• 10 test tubes (8 for knockout strains, 1 for wild type positive control, and 1 for a negative control w/o yeast)
• 1 tube of each (8 different ones tested in this experiment: YCR100C, YJL043W, YEL035C, YMR090W, YPL068C, YCL002C, YGL235W, YFL064C) knockout strain tested
• 1 tube of control yeast: BY4735
• 30 autosampler vials
Equipment
• High Performance Laser Chromatography (HPLC)
Instrument-Shimadzu UFLC
Column- Phenomenex Rezex ROA- Organic H+ 8%, 300x7.8mm
Detector: Shimadzu RID-10A refractive index detector
• Incubator
Set to- 30 degrees C
• Centrifuge
Protocol
1. Make 1-L Fermentation Media in 1-L PYREX Bottle
a. add 1.71 g of Yeast Nitrogen base (Sunrise Science)
b. add .65 g Complete Supplement Mixture (Sunrise Science)
c. add 5 g of ammonium sulfase
d. add 10 ml of Histodine (200mg/100ml)
e. add 10 ml of Uracil(200mg/100ml)
f. add 10 ml of Leucine (1.2g/100ml)
g. fill bottle with distilled water to 1-L mark
h. autoclave media
i. add 41.5 ml of 50% w/v sucrose solution to 1-L fermentation media in order to have 2% sucrose solution in fermentation media
2. Set up Fermentation
a. Add 10 ml of fermentation media to each test tube
b. Add 1 ml of yeast each test tube (each test tube gets 1 ml of a different yeast strain. One tube gets no yeast this is negative control)
c. vortex briefly
d. Transfer 1.5 ml out of each tube into a centrifuge tube and freeze- This is T0 sample
e. Cap tubes with test tube cap
f. Place tubes in incubator at 30oC
3. Extraction- vortex each tube briefly then take 1.5 ml of each fermentation system at
a. T2 Day 2- place extractd in freezer, fermentation system back in incubator
b. T7 Day 7- place in freezer until ready to centrifuge
4. Prepare for HPLC
a. Centrifuge all centrifuge tubes of fermented yeast from T0, T2, and T7 for 10 minutes at 15,000 rpms
b. Extract 1 ml of liquid only from top of each centrifuge tube (be sure to not extract yeast that has accumulated at the bottom)
c. Place 1 ml of liquid into 1.5 ml autosampler tube
5. Send all autosampler vials through HPLC
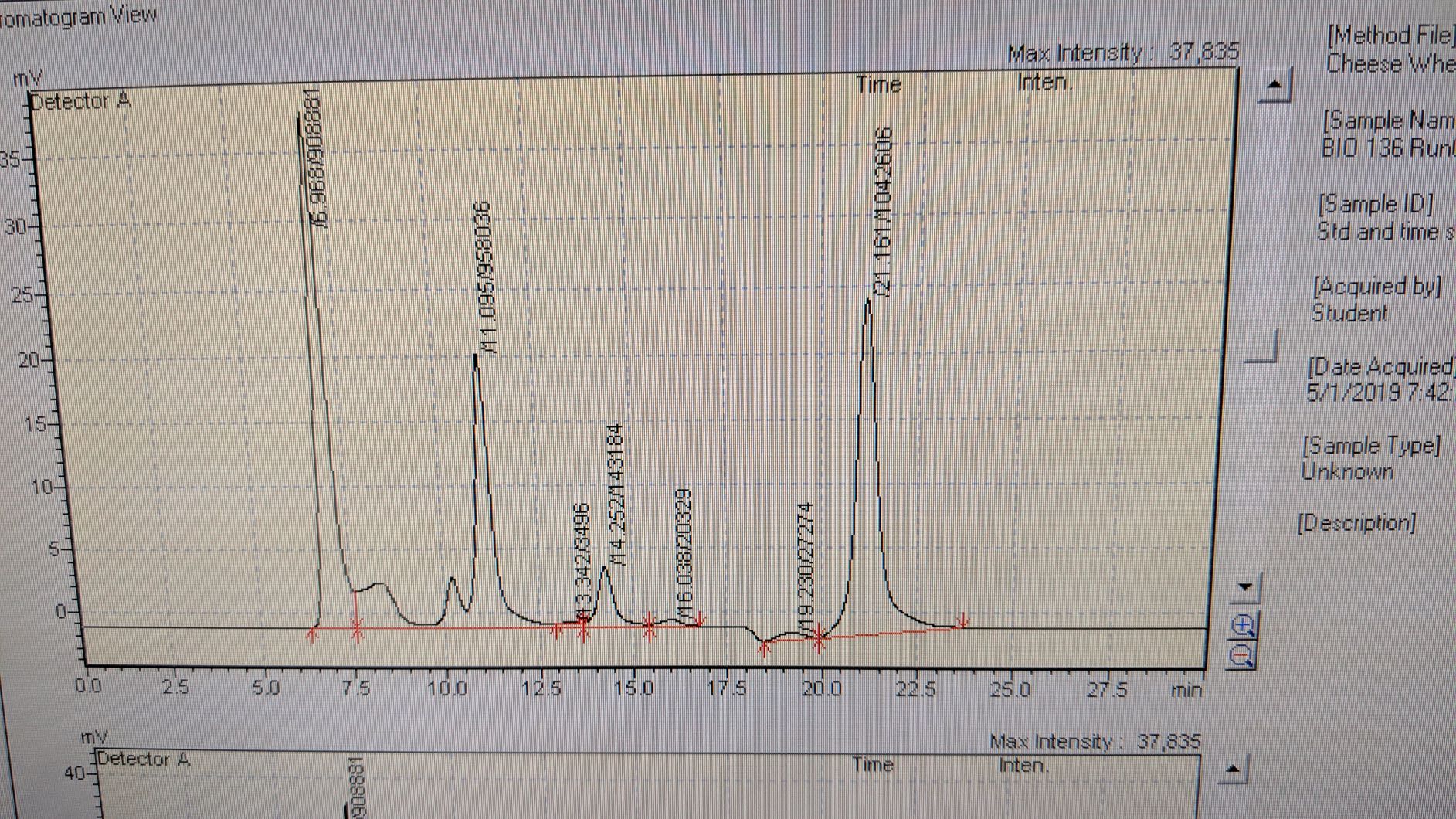
HPLC Shows peaks for store bought sucrose and ethanol. The double-peak at 10 and 11 minutes is the sucrose peak. Note the sucrose peaks will not be one peak since the standards were made with store bought sugar. Peaks for ethanol should appear around 21 minutes.