UW-Stout/Ethanol SP21
Contents
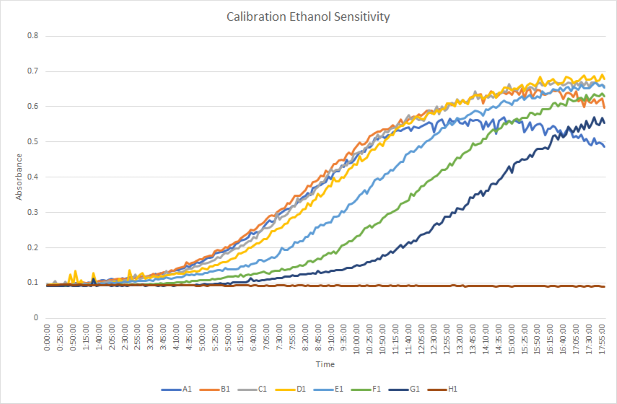
Calibration Experiment Protocol
Introduction
This protocol will be measuring the growth curve of a single yeast strain at eight different concentrations of ethanol. One of the eight concentrations will contain no ethanol and act as a control for the rest of our results to be compared to. This process will determine which concentration of ethanol is most appropriate based on yeast growth in relation to ethanol sensitivity.
Safety
Ethanol can be a toxic chemical under certain concentrations and combinations of organisms. It is also very flammable, so it should be handled and treated with caution as well as, one should not touch their eyes with contents of ethanol on their skin. Proper safety measures such as a lab coat, gloves, and safety glasses may be necessary to protect your lab results and personal health.
Materials/Equipment
- Corning COSTAR96-well clear flat-bottom assay plate
- Sterile H2O
- Wild-type yeast in 2x synthetic complete media at an OD600 of 0.1-0.2 (50ul per well)
- 100% ethanol (0ul, 0.3ul, 1ul, 3ul, 6ul, 9ul, 12ul, 24ul)
- Molecular Devices SpectaMax Plus 384 Microplate Reader
- Pipettors (1000ul, 200ul, 20ul, 10ul)
- Pipettor tips (blue, green, red)
- Centrifuge tube
- Vent hood
Protocol
- Set up all materials and necessary equipment under the vent hood.
- Vortex the yeast culture briefly to resuspend the yeast cells.
- Insert 900ul of water and 100ul of 100% ethanol into a centrifuge tube.
- Use the 10% ethanol just created to complete Well 2 (3ul 10% ethanol, 47ul water=0.3ul 100% ethanol, 49.7ul water).
- Set up eight wells according to the details labeled Well Components.
- Set up the plate reader as follows: Temperature: 30°C, Mode: Kinetic, Wavelength: 600 nm, Interval: 10 minutes, Total run time: 12 hours, Shake before read: 30 seconds
- Transfer the assay plate to the reader and read for 24 hours.
- Observe the Molecular Devices SpectaMax Plus 384 Microplate Reader results.
Well Components
Well 1 (control)
- 0 ul ethanol
- 50 ul yeast
- 50 ul water
Well 2
- 0.3 ul ethanol
- 50 ul yeast
- 49.7 ul water
Well 3
- 1 ul ethanol
- 50 ul yeast
- 49 ul water
Well 4
- 3 ul ethanol
- 50 ul yeast
- 47 ul water
Well 5
- 6 ul ethanol
- 50 ul yeast
- 44 ul water
Well 6
- 9 ul ethanol
- 50 ul yeast
- 41 ul water
Well 7
- 12 ul ehanol
- 50 ul yeast
- 38 ul water
Well 8
- 24 ul ethanol
- 50 ul yeast
- 26 ul water
Data
When observing the data collected from this protocol, a concentration of 9% ethanol was determined to be the most appropriate concentration that showed successful growth levels. The 0.3% concentration of ethanol had no growth of the cells, whereas the 24% concentration had too much growth to the point where cells ended up dying. Therefore, a concentration in-between these two concentrations expressed a more realistic growth curve that could be used for the experimental experiment.
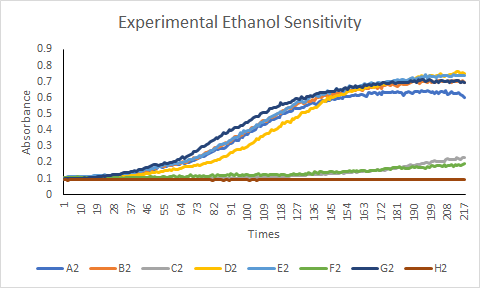
KO Genes Experimental Protocol
Introduction
This protocol will be measuring the growth curve of a single ethanol concentration on six different classmates yeast strains and one of my yeast strain (YKL121W). There will also be one controlled well that does not contain any ethanol. This process will determine which yeast strain has the least and greatest sensitivity to ethanol.
Safety
Ethanol can be a toxic chemical under certain concentrations and combinations of organisms. It is also very flammable, so it should be handled and treated with caution as well as, one should not touch their eyes with contents of ethanol on their skin. Proper safety measures such as a lab coat, gloves, and safety glasses may be necessary to protect your lab results and personal health.
Materials/Equipment
- Corning COSTAR96-well clear flat-bottom assay plate
- Sterile H2O
- Wild-type yeast in 2x synthetic complete media at an OD600 of 0.1-0.2
- 6 yeast strains (YOR387C, YKL121W, YBR225W, YNL018C, YLR426W, YGL138C, YKL121W)
- 100% ethanol (9ul per well)
- Molecular Devices SpectaMax Plus 384 Microplate Reader
- Pipettors (1000ul, 200ul, 20ul, 10ul)
- Pipettor tips (blue, green, red)
- Centrifuge tube
- Vent hood
Protocol
- Set up all materials and necessary equipment under the vent hood.
- Vortex the yeast culture briefly to resuspend the yeast cells.
- Insert 900ul of water and 100ul of 100% ethanol into a centrifuge tube.
- Use the 10% ethanol just created to complete Well 2 (3ul 10% ethanol, 47ul water=0.3ul 100% ethanol, 49.7ul water).
- Set up seven wells according to the details labeled Well Components.
- Set up the plate reader as follows: Temperature: 30°C, Mode: Kinetic, Wavelength: 600 nm, Interval: 10 minutes, Total run time: 12 hours, Shake before read: 30 seconds
- Transfer the assay plate to the reader and read for 24 hours.
- Observe the Molecular Devices SpectaMax Plus 384 Microplate Reader results.
Well Components
Well 1 (Wild-Type)
- 9 ul ethanol
- 50 ul yeast
- 41 ul water
Well 2 (YOR387C)
- 9 ul ethanol
- 50 ul yeast
- 41 ul water
Well 3 (YKL121W)
- 9 ul ethanol
- 50 ul yeast
- 41 ul water
Well 4 (YBR225W)
- 9 ul ethanol
- 50 ul yeast
- 41 ul water
Well 5 (YNL018C)
- 9 ul ethanol
- 50 ul yeast
- 41 ul water
Well 6 (YLR426W)
- 9 ul ethanol
- 50 ul yeast
- 41 ul water
Well 7 (YGL138C)
- 9 ul ethanol
- 50 ul yeast
- 41 ul water
Data
When observing the data collected from this protocol, it was determined that many of the strains of yeast tested were sensitive to ethanol. The strains that experienced cell death when stressed with 9% concentration of ethanol included YOR387C, YKL121W, YBR225W, YNL018C, and YGL138C. While the two other strains YLR426W and YKL121W had successful cell growth expressed from the growth curve.