UW-Stout/Ammonium Sulfate SP21
Contents
Materials
- Yeast Media Ingredients
- Yeast nitrogen base (YNB)
- CSM His-Leu-Ura
- Water
- 40% w/v Glucose Solution
- P1000/P200/P20/P10 Micropipettes with corresponding tips
- 1.5 ml Microcentrifuge tubes
- 96 well plate
- Sterile Water
- Media glassware with cap
- 50 ml beaker
Equipment
Preparation
Ammonium Sulfate Lacking Solution
First the solution with out ammonium sulfate needs to be made. Mix the following ingredients into a piece of glassware with a removable cap. The order does not matter, though it is good to start with adding the water.
- 0.342g YNB
- 0.13g CSM
- 2 ml of 100x His-Leu-Ura solutions
- 95 ml of water
Once mixed thoroughly, autoclave for 30 minutes. Once glassware is reasonably cool, add 10 ml of glucose.
Prepare the 5g/ml Ammonium Sulfate Solution and the 100x Dilution
In a 50 ml beaker mix the following
- 0.05g of Ammonium sulfate
- 10 ml of water
Prepare the 100x dilution by mixing the following in 150 ml beaker
- 1 ml of 5g/ml solution
- 99 ml of water
Will only need a filled microcentrifuge tube of each so transfer the adequate amount for easier handling of solutions.
Switching Yeast from Ammonium Sulfate Containing Media to Lacking Media
To separate the yeast cells from media, follow these steps. NOTE: When handling the live yeast cells operate under the hood!
- Vortex stock solution of cells.
- Transfer 1 ml of cells into microcentrifuge tube.
- Centrifuge at 1000 rcf for 2 minutes.
- Pipette off the supernatant leaving the pellet (the cells).
- Apply a PBS solution rinse and filter out PBS
- Transfer 1 ml of the ammonium sulfate lacking media prepared earlier into the microcentrifuge tube.
- Vortex to resuspend the cells.
Wild Yeast Calibration Plating
In your designated well column, for example #7, pipette the following amounts into each of the corresponding rows.
A: 50ul Cells, 40ul H20, 10ul Ammonium Sulfate solution
B: 50ul Cells, 46ul H20, 4ul Ammonium Sulfate solution
C: 50ul Cells, 48ul H20, 2ul Ammonium Sulfate solution
D: 50ul Cells, 49ul H20, 1ul Ammonium Sulfate solution
E: 50ul Cells, 49.6ul H20, 0.4ul Ammonium Sulfate solution
F: 50ul Cells, 49.8ul H20, 0.2ul Ammonium Sulfate solution
G: 50ul Cells, 49ul H20, 1ul 100x Ammonium Sulfate solution
H: 50ul Cells, 50ul H20
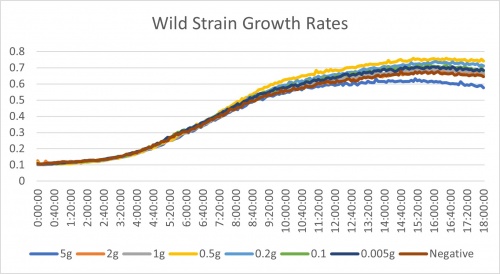
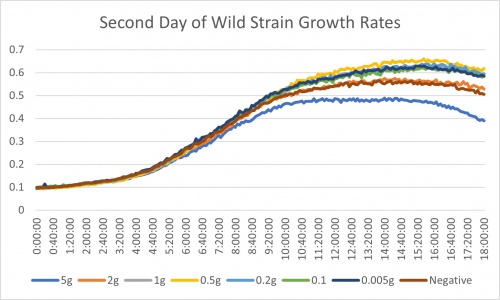
Place into the incubator for 24 hours and examine plate in the plate reader. Results are listed below.
Analysis of Calibration Protocol
After analyzing the results from the PBS rinse modification (day 2 results) we made a few decisions. Ultimately we are moving forward with testing against KO strains. We did not see any significant difference between the data from the first attempt of the experiment. The trends of the growth rates are all similar. It is hard to tell the effectiveness of each of the treatments but we did conclude one finding. The 0.5g, 5g, and negative control all stayed in the same relative position in the trends. 0.5g appeared at the top with the fastest growth rate. Negative control remained at a surprisingly neutral growth rate. 5g showed to be the slowest. These values of change are very miniscule though. The reason we are moving forward is one due to time, but also it could be that one of the genes knocked out could be important in this process of low nitrogen growth. If this is the case, we should see significantly lower growth rates in these strains. It is important to make sure that these lower growth rates are not just due to the gene being gone. It is expected that they would be lower. We need substantial evidence that the gene being knocked out is linked to nitrogen starvation.
Knock Out Strain Plating
This is set up for 3 treatments at once so take care when pipetting. You will have 3 designated columns, for example use #1-3, and will use a different strain in each of the rows. Order your plate as the information below shows and transfer the correct volumes.
Each Row will be using a different yeast strain. Be sure to be pipetting the correct yeast strains into the correct rows.
Column #1: Treatment 5g/ml Ammonium Sulfate
Wild Row A: 50ul Wild yeast Cells, 40ul H2O, 10ul Ammonium Sulfate
KO1 Row B: 50ul KO1 yeast Cells, 40ul H2O, 10ul Ammonium Sulfate
KO2 Row C: 50ul KO2 yeast Cells, 40ul H2O, 10ul Ammonium Sulfate
KO3 Row D: 50ul KO3 yeast Cells, 40ul H2O, 10ul Ammonium Sulfate
KO4 Row E: 50ul KO4 yeast Cells, 40ul H2O, 10ul Ammonium Sulfate
KO5 Row F: 50ul KO5 yeast Cells, 40ul H2O, 10ul Ammonium Sulfate
KO6 Row G: 50ul KO6 yeast Cells, 40ul H2O, 10ul Ammonium Sulfate
Column #2: Treatment: 0.5g/ml Ammonium Sulfate
Wild Row A: 50ul Wild yeast Cells, 49ul H2O, 1ul Ammonium Sulfate
KO1 Row B: 50ul KO1 yeast Cells, 49ul H2O, 1ul Ammonium Sulfate
KO2 Row C: 50ul KO2 yeast Cells, 49ul H2O, 1ul Ammonium Sulfate
KO3 Row D: 50ul KO3 yeast Cells, 49ul H2O, 1ul Ammonium Sulfate
KO4 Row D: 50ul KO4 yeast Cells, 49ul H2O, 1ul Ammonium Sulfate
KO5 Row F: 50ul KO5 yeast Cells, 49ul H2O, 1ul Ammonium Sulfate
KO6 Row G: 50ul KO6 yeast Cells, 49ul H2O, 1ul Ammonium Sulfate
Column #3: Treatment: Negative Control
Wild Row A: 50ul Wild yeast Cells, 50ul H2O
KO1 Row B: 50ul KO1 yeast Cells, 50ul H2O
KO2 Row C: 50ul KO2 yeast Cells, 50ul H2O
KO3 Row D: 50ul KO3 yeast Cells, 50ul H2O
KO4 Row E: 50ul KO4 yeast Cells, 50ul H2O
KO5 Row F: 50ul KO5 yeast Cells, 50ul H2O
KO6 Row G: 50ul KO6 yeast Cells, 50ul H2O
Place into the incubator for 24 hours and examine plate in the plate reader.