Difference between revisions of "UW-Stout/pH Acid SP22"
| Line 31: | Line 31: | ||
1. To create a buffer solution at a specific pH, you must combine x ml of citric acid monohydrate with y ml of disodium phosphate and shake well | 1. To create a buffer solution at a specific pH, you must combine x ml of citric acid monohydrate with y ml of disodium phosphate and shake well | ||
pH x ml 0.1M-Citric Acid y ml 0.2M-Na2HPO4 | pH x ml 0.1M-Citric Acid y ml 0.2M-Na2HPO4 | ||
| − | + | [[Image:calibration amount.jpg]] | |
| − | |||
| − | |||
| − | |||
| − | |||
==Calibration Experiment protocol== | ==Calibration Experiment protocol== | ||
Revision as of 12:07, 28 April 2022
Contents
Introduction
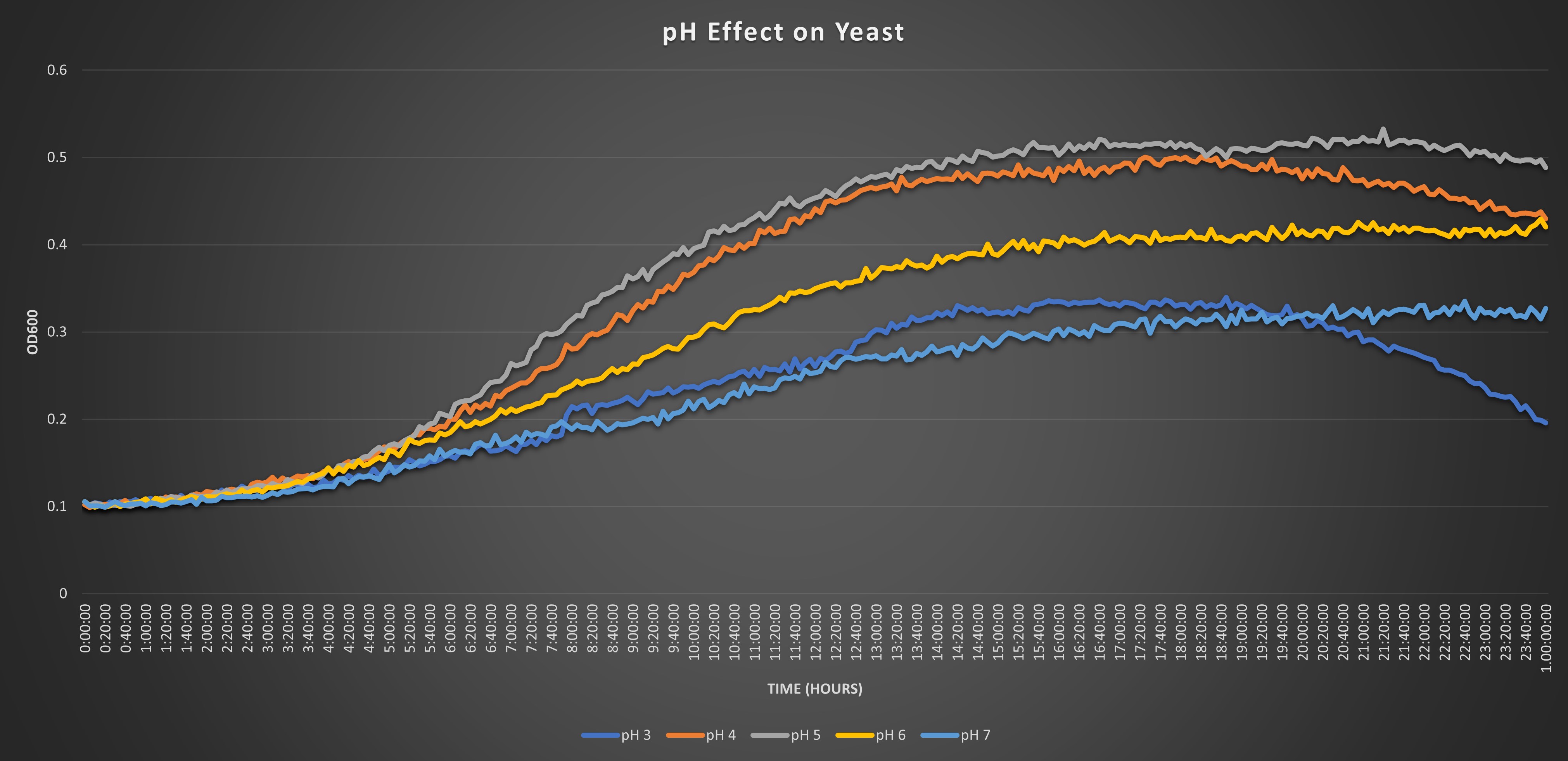
Our protocol is designed to determine if the knocked-out gene affects or does not affect the growth of our yeasts in environments of pH 7. The pH of 7 was predetermined in a pilot experiment to be a pH that would stress the cell’s growth without killing all the cells. A citric acid monohydrate buffer was used to maintain a pH of 7 in the growth environments throughout the entire growth process.
Materials/Equipment
• Citric Acid Monohydrate • Disodium Phosphate • De-ionized Water • Micropipette • 15ml tube (3x) • 96-well clear flat-bottom assay plate • Micropipette tips • Gloves • Incubator set to 30 C • Scale • Sterile cabinet • pH test strips • Yeast strains
Procedure
Preparing Buffer Stock Solutions (0.1M citric acid, 0.2M disodium phosphate) 1. To obtain 0.1M of citric acid monohydrate, combine .21g of in 10ml of de-ionized water 2. To obtain 0.2M of disodium phosphate, combine .28g in 10 ml de-ionized water
Preparing Buffer Solution
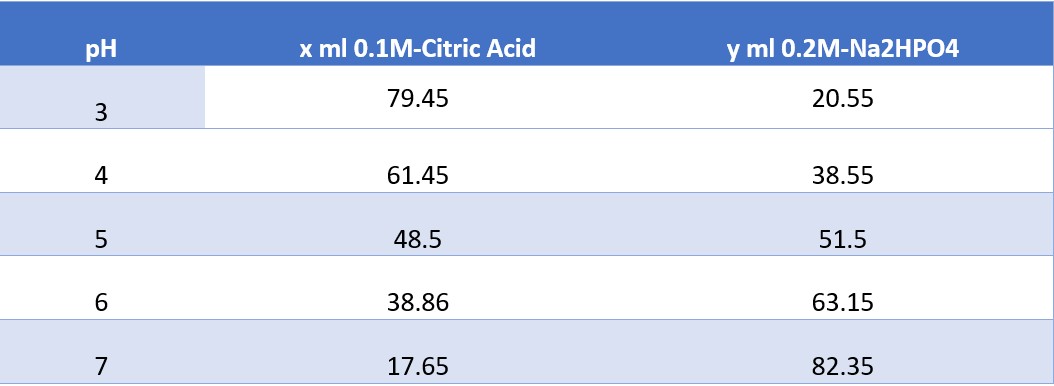
1. To create a buffer solution at a specific pH, you must combine x ml of citric acid monohydrate with y ml of disodium phosphate and shake well
pH x ml 0.1M-Citric Acid y ml 0.2M-Na2HPO4
Calibration Experiment protocol
Final Experiment protocol
Addition of Buffer to Yeast 1. Vortex wild type strain and knock out strains 2. Add 50ul of each knock out strain to own each well 3. Add 50ul of pH buffer to each subsequent well
Preparing Plate Reader
• Temperature: at 30 degrees Celsius • Mode: Kinetic • Read: 600 n • Intervals: 5 minutes • Total run time: 24 hours • Shake before read: 30 seconds • Transfer the assay plate to the reader and run for 24 hours