Difference between revisions of "UW-Stout/UV Light SP21"
(→Calibration Results) |
(→Calibration Results) |
||
| Line 24: | Line 24: | ||
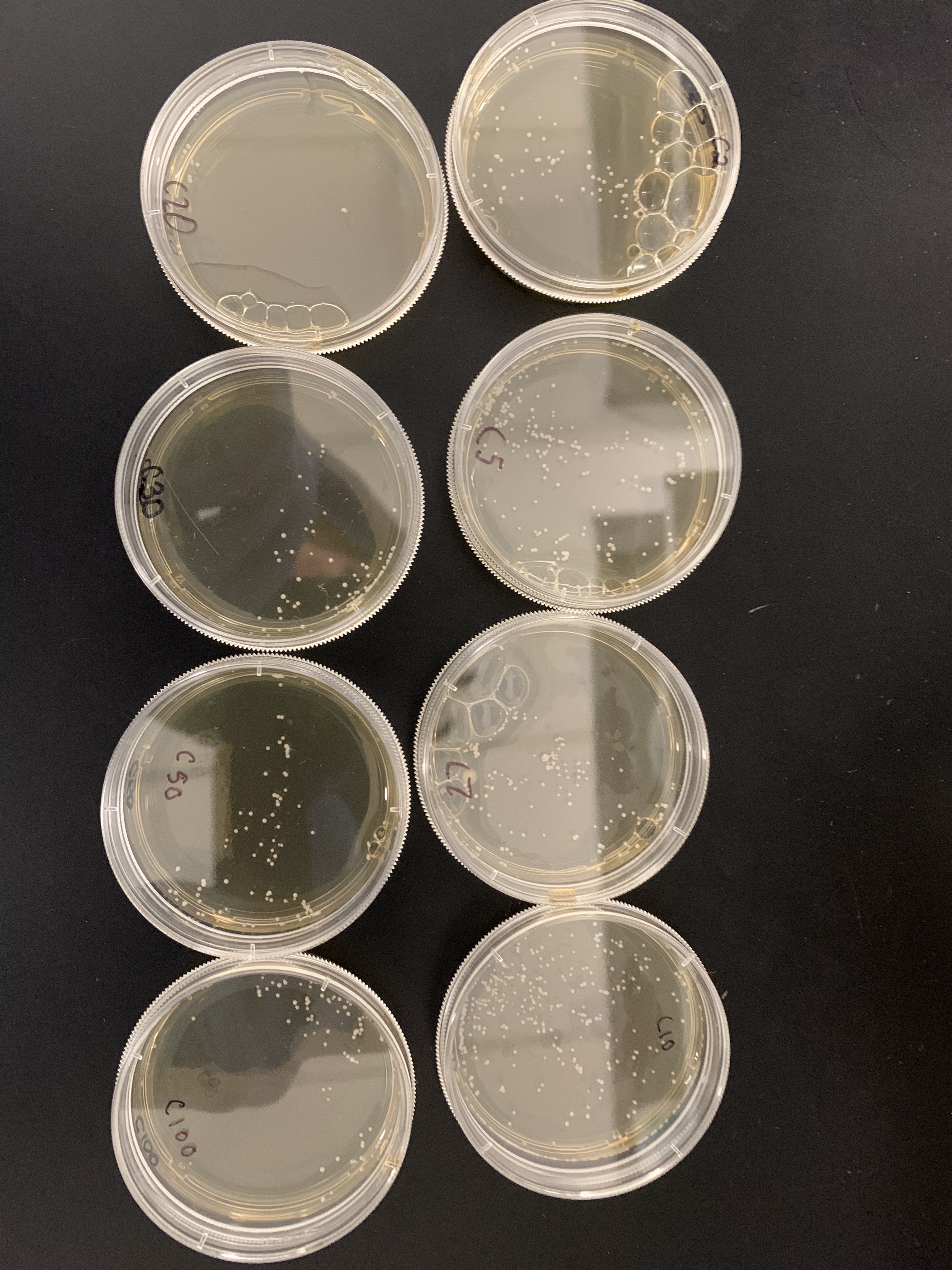
==Calibration Results== | ==Calibration Results== | ||
| − | [[4A1B2FAF-1C83-4A7A-AE89-FFC5BFBE7D35.jpeg]] | + | [[Image:4A1B2FAF-1C83-4A7A-AE89-FFC5BFBE7D35.jpeg|4A1B2FAF-1C83-4A7A-AE89-FFC5BFBE7D35.jpeg]] |
==Knock-out Protocol== | ==Knock-out Protocol== | ||
==Knock-out Results== | ==Knock-out Results== | ||
Revision as of 09:50, 27 April 2021
Contents
Materials
- Cell 60 mm Culture Dish, containing 6 ml of agar
- Phosphate Buffered Saline (PBS)
- Glycerol stocks of yeast strains
- 15ml centrifuge tubes
Equipment
- Incubator set to 30°C.
- Bachur & Associates Sanata Clara, CA 95050 Model LS-100-3 UV Light Exposeure System
- P1000 and P10 Micro-pipettes
Calibration Protocol
- Fill a 15ml centrifuge tube with 9,990ul of PBS.
- Vortex yeast stock to resuspend the yeast cells.
- Pipette 10ul of wild yeast stock into the 9,990ul of PBS to create a dilution containing 2 yeast cells per microliter.
- Vortex dilution and prepare 7 plates with 50ul of the wild yeast dilution for about 100 yeast cells per plate.
- Label each plate individually 0, 500, 600, 700, 800, 900 and 1000 for the number of seconds each plate will be exposed to the UV light.
- Set up the UV light exposure system:
- 400 watts
- desired time increment
- Run yeast plates (without plate top) under the UV light for their respective times in seconds.
- Place plates upside down in dark incubator set to 30°C for 48 hours.
- Count number of colonies on each plate using the 0 second plate as your control to compare to. Based on how many colonies there are on each plate, determine the time frame that killed roughly 50% of the yeast cells.