Difference between revisions of "UW-Stout/Caffeine FA19"
(→Protocol for Stressing the Knockout Strands with Caffeine) |
|||
| (8 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
*Syringes and purifier | *Syringes and purifier | ||
*96 Well assay plate | *96 Well assay plate | ||
| + | *Wild type yeast cells | ||
'''Procedure''' | '''Procedure''' | ||
| Line 16: | Line 17: | ||
#Fill the wells with different concentrations of caffeine solution. | #Fill the wells with different concentrations of caffeine solution. | ||
#After the wells are completed, place the 96 Well assay plate into the SpectraMax Plus 384 Microplate Reader. Data will be collected every 5 minutes for the next 24 hours. | #After the wells are completed, place the 96 Well assay plate into the SpectraMax Plus 384 Microplate Reader. Data will be collected every 5 minutes for the next 24 hours. | ||
| − | #Once results are computed the data curve should be put on the growth data curve | + | #Once results are computed the data curve should be put on the growth data curve. |
| − | Volume of the Stock solution of 10mM ( | + | |
| − | 0 | + | {| class="wikitable" |
| − | 50 | + | |+ Concentrations of Caffeine to Yeast Cells |
| − | 50 | + | |- |
| − | - | + | ! Concentration of solution added to yeast (μM) |
| − | 0 | + | ! Volume of solution added to yeast (μl) |
| − | 0.10 | + | ! Water added to the solution (μl) |
| − | 50 | + | ! Volume of the Stock solution of 100mM (μl) |
| − | 49 | + | ! Volume of the Stock solution of 10mM (μl) |
| − | - | + | |- |
| − | + | | 0 | |
| − | 0.25 | + | | 50 |
| − | 50 | + | | 50 |
| − | 47.5 | + | | - |
| − | - | + | | 0 |
| − | 2.5 | + | |- |
| − | 0.50 | + | | 0.10 |
| − | 50 | + | | 50 |
| − | 45 | + | | 49 |
| − | - | + | | - |
| − | 5 | + | | 0 |
| − | 1.0 | + | |- |
| − | 50 | + | | 0.25 |
| − | 40 | + | | 50 |
| − | - | + | | 47.5 |
| − | 10 | + | | - |
| − | 2.5 | + | | 2.5 |
| − | 50 | + | |- |
| − | 47.5 | + | | 0.50 |
| − | 2.5 | + | | 50 |
| − | - | + | | 45 |
| − | 5.0 | + | | - |
| − | 50 | + | | 5 |
| − | 45 | + | |- |
| − | 5 | + | | 1.0 |
| − | - | + | | 50 |
| − | 8.0 | + | | 40 |
| − | 50 | + | | - |
| − | 42 | + | | 10 |
| − | 8 | + | |- |
| − | - | + | | 2.5 |
| − | 10 | + | | 50 |
| − | 50 | + | | 47.5 |
| − | 40 | + | | 2.5 |
| − | 10 | + | | - |
| − | - | + | |- |
| − | 15 | + | | 5.0 |
| − | 50 | + | | 50 |
| − | 35 | + | | 45 |
| − | 15 | + | | 5 |
| − | - | + | | - |
| − | 20 | + | |- |
| − | 50 | + | | 8.0 |
| − | 30 | + | | 50 |
| − | 20 | + | | 42 |
| − | - | + | | 8 |
| − | 50 | + | | - |
| − | 50 | + | |- |
| − | 0 | + | | 10 |
| − | 50 | + | | 50 |
| − | - | + | | 40 |
| + | | 10 | ||
| + | | - | ||
| + | |- | ||
| + | | 15 | ||
| + | | 50 | ||
| + | | 35 | ||
| + | | 15 | ||
| + | | - | ||
| + | |- | ||
| + | | 20 | ||
| + | | 50 | ||
| + | | 30 | ||
| + | | 20 | ||
| + | | - | ||
| + | |- | ||
| + | | 50 | ||
| + | | 50 | ||
| + | | 0 | ||
| + | | 50 | ||
| + | | - | ||
| + | |} | ||
| + | |||
| + | |||
| + | ===Results form Caffeine Yeast Expirement=== | ||
| + | |||
| + | |||
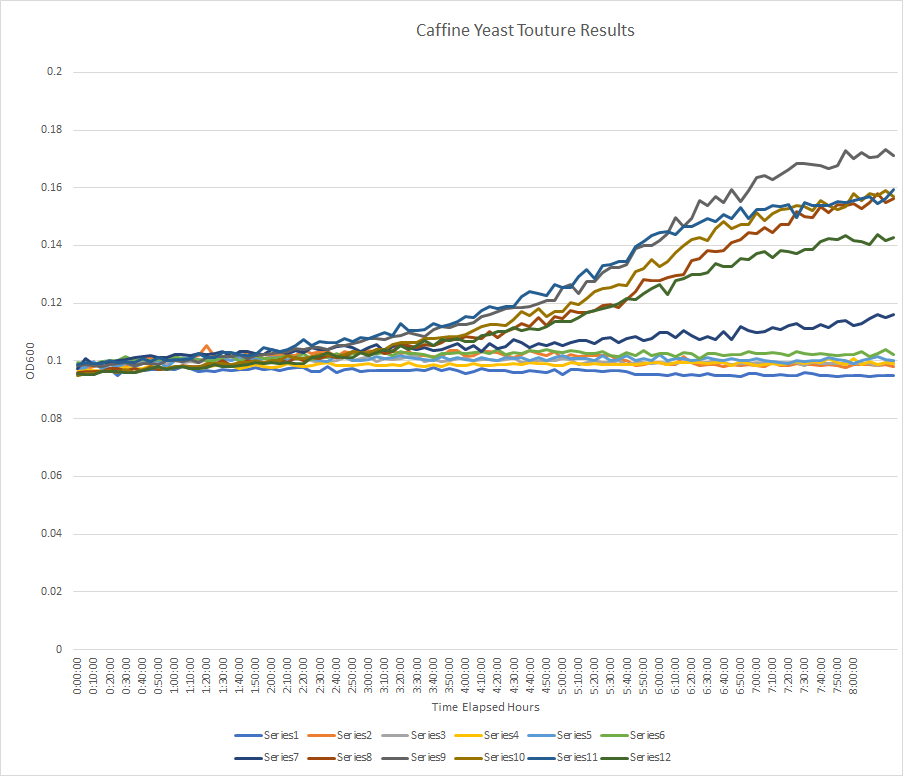
| + | [[Image:megangraph1.png]] | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+ Caffeine Concentration in Graph: | ||
| + | |- | ||
| + | !Series | ||
| + | !Concentration in μM | ||
| + | |- | ||
| + | | 1 | ||
| + | | 50 | ||
| + | |- | ||
| + | | 2 | ||
| + | | 20 | ||
| + | |- | ||
| + | | 3 | ||
| + | | 15 | ||
| + | |- | ||
| + | | 4 | ||
| + | | 10 | ||
| + | |- | ||
| + | | 5 | ||
| + | | 8.0 | ||
| + | |- | ||
| + | | 6 | ||
| + | | 5.0 | ||
| + | |- | ||
| + | | 7 | ||
| + | | 2.5 | ||
| + | |- | ||
| + | | 8 | ||
| + | | 1.0 | ||
| + | |- | ||
| + | | 9 | ||
| + | | 0.50 | ||
| + | |- | ||
| + | | 10 | ||
| + | | 0.25 | ||
| + | |- | ||
| + | | 11 | ||
| + | | 0.10 | ||
| + | |- | ||
| + | | 12 | ||
| + | | 0.0 | ||
| + | |} | ||
| + | |||
| + | |||
| + | After the data was reviewed the concentration that was picked to run the rest of the experiments on was 0.50μM. It had the highest growth rate with caffeine, which was the reason why it was selected. This concentration when compared to the control, had the highest growth rate. This was very surprising because the caffeine was supposed to kill the yeast cells but instead, it increased the OD600 on the wild type yeast cells. | ||
| + | |||
| + | |||
| + | ===Protocol for Stressing the Knockout Strands with Caffeine=== | ||
| + | |||
| + | '''Materials''' | ||
| + | *Sterile H2O | ||
| + | *Yeast Strands | ||
| + | *96 Well Plate | ||
| + | *Caffeine Stock Solution | ||
| + | |||
| + | |||
| + | '''Protocol:''' | ||
| + | |||
| + | #Create a sufficient amount of 10μM stock solution, mix 72μl of sterile H2O with 8μl of 100μM stock solution, in order to replicate series 9 from the previous experiment. | ||
| + | #Add 45μl of sterile H2O to each of the wells. | ||
| + | #Add 5μl of diluted caffeine solution to each well. | ||
| + | #Then add 50μl of each strand of yeast being tested. | ||
| + | #After the solution in the wells is completed put the 96 well plate into the SpectaMax Plus 384 Microplate Reader where the data will be collected every 5 minutes for the next 24 hours. | ||
Latest revision as of 07:22, 12 December 2019
Caffeine Dilution and Concentration of Caffeine Protocol
Materials
- 0.22g Caffeine
- Sterile H2O
- Syringes and purifier
- 96 Well assay plate
- Wild type yeast cells
Procedure
- Make a 100μM stock solution of caffeine and H2O.
- Take 0.1942 grams of caffeine to 10ml of H2O to form a stock solution of 100C.
- Make another stock solution of 10μM of caffeine and H2O.
- Dilute the 100μM stock solution of 10μM stock solution by adding 4μl of 100μM of the stock solution and 36μl sterile H20.
- Filter the caffeine solution using the syringes and purifier then put in microfuge tubes, freeze at -20°C.
- Dilute the 100μM stock solution into the 12 concentrations using the table below.
- Fill the wells with different concentrations of caffeine solution.
- After the wells are completed, place the 96 Well assay plate into the SpectraMax Plus 384 Microplate Reader. Data will be collected every 5 minutes for the next 24 hours.
- Once results are computed the data curve should be put on the growth data curve.
| Concentration of solution added to yeast (μM) | Volume of solution added to yeast (μl) | Water added to the solution (μl) | Volume of the Stock solution of 100mM (μl) | Volume of the Stock solution of 10mM (μl) |
|---|---|---|---|---|
| 0 | 50 | 50 | - | 0 |
| 0.10 | 50 | 49 | - | 0 |
| 0.25 | 50 | 47.5 | - | 2.5 |
| 0.50 | 50 | 45 | - | 5 |
| 1.0 | 50 | 40 | - | 10 |
| 2.5 | 50 | 47.5 | 2.5 | - |
| 5.0 | 50 | 45 | 5 | - |
| 8.0 | 50 | 42 | 8 | - |
| 10 | 50 | 40 | 10 | - |
| 15 | 50 | 35 | 15 | - |
| 20 | 50 | 30 | 20 | - |
| 50 | 50 | 0 | 50 | - |
Results form Caffeine Yeast Expirement
| Series | Concentration in μM |
|---|---|
| 1 | 50 |
| 2 | 20 |
| 3 | 15 |
| 4 | 10 |
| 5 | 8.0 |
| 6 | 5.0 |
| 7 | 2.5 |
| 8 | 1.0 |
| 9 | 0.50 |
| 10 | 0.25 |
| 11 | 0.10 |
| 12 | 0.0 |
After the data was reviewed the concentration that was picked to run the rest of the experiments on was 0.50μM. It had the highest growth rate with caffeine, which was the reason why it was selected. This concentration when compared to the control, had the highest growth rate. This was very surprising because the caffeine was supposed to kill the yeast cells but instead, it increased the OD600 on the wild type yeast cells.
Protocol for Stressing the Knockout Strands with Caffeine
Materials
- Sterile H2O
- Yeast Strands
- 96 Well Plate
- Caffeine Stock Solution
Protocol:
- Create a sufficient amount of 10μM stock solution, mix 72μl of sterile H2O with 8μl of 100μM stock solution, in order to replicate series 9 from the previous experiment.
- Add 45μl of sterile H2O to each of the wells.
- Add 5μl of diluted caffeine solution to each well.
- Then add 50μl of each strand of yeast being tested.
- After the solution in the wells is completed put the 96 well plate into the SpectaMax Plus 384 Microplate Reader where the data will be collected every 5 minutes for the next 24 hours.