UW-Stout/Hydrogen Peroxide FA22
Contents
Introduction
We are using hydrogen peroxide to displaying varying growth of yeast cells. In this experiment, we tested many different concentrations then many different yeast cells with different genes knocked out.
Materials
- 5000µM hydrogen peroxide solution
- 3% hydrogen peroxide
- Sterile water for dilutions
- Wild-type yeast in 2x synthetic complete media at an OD600 of 0.1-0.2
- Yeast with different knocked out genes (except wild type, the gene listed is the one knocked out)
- BY4735 (wild type)
- YJL133C-A
- YER186C
- YER076C
- YHR033W
- YCR095C
Equipment
- Molecular Devices SpectraMax Plus 384 Microplate Reader
- Assay plate
- Micropettes
Pilot Experiment
Protocol
- Vortex the BY4735 yeast culture briefly to resuspend the yeast cells.
- Create dilutions from the stock hydrogen peroxide
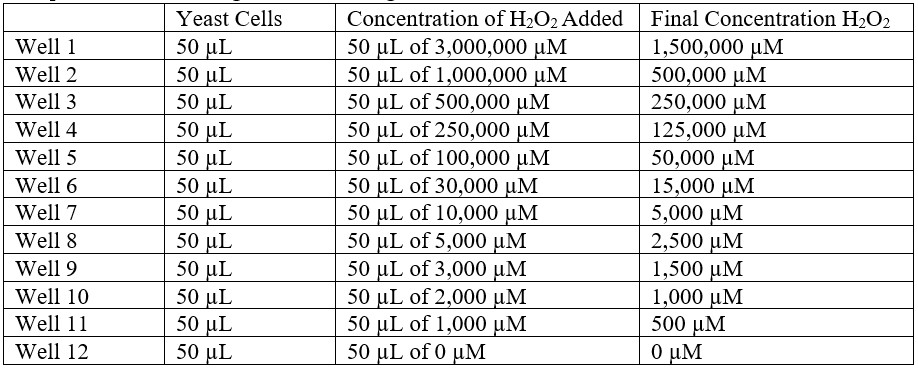
- Set up 12 wells in the assay plate according to the following table:
- Set up the plate reader as follows:
- Temperature: 30°C
- Mode: Kinetic
- Wavelength: 600 nm
- Interval: 5 minutes
- Total run time: 24 hours
- Shake before read: 30 seconds
- Transfer the assay plate to the reader and read for 24 hours
Results
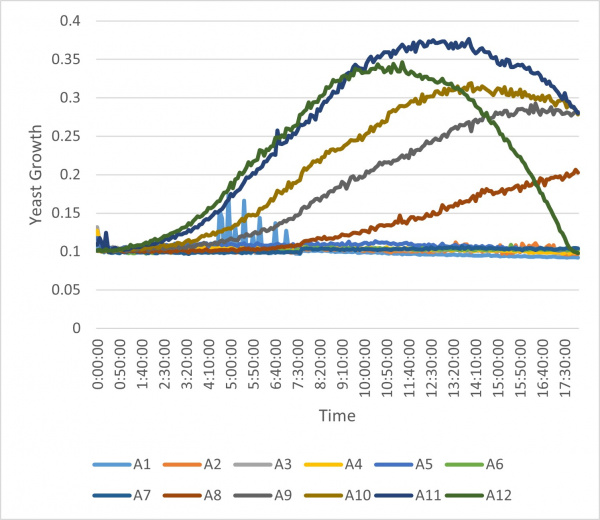
The following growth curve chart was generated from the data.
The well number corresponds to the concentration as shown in the chart above. From the graph, we decided to use the concentration from well A8. This corresponds to the 5000µM hydrogen peroxide solution. This is the concentration we decided to use for the knockout experiment because it demonstrated an effect on the growth of wild type yeast without killing majority of the yeast cells.
Knockout Experiment
- Vortex yeast cells briefly to resuspend cells
- Set up the plate as follows with 50µL of yeast and 50µL of 5000µM hydrogen peroxide
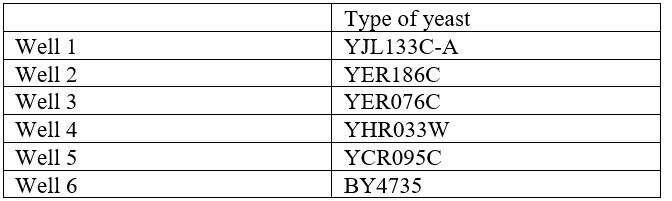
- Use the following table to set up the wells:
- Set up the plate reader as follows
- Temperature: 30°C
- Mode:Kinetic
- Wavelength: 600 nm
- Interval: 5 minutes
- Total run time: 24 hours
- Shake before read: 30 seconds
- Transfer the assay plate to the read and read for 24 hours