UW-Stout/Nonionic Detergent SP22
Pilot experiment:
Testing NLS effect on WT yeast cells growth
1. Introduction: In this experiment, we will be testing the effect of N-Lauroylsarcosine on the speed of growth of wild type yeast cells. Our objective is to find the concentration of NLS needed to affect the yeast cell growth without killing them all.
2. Materials: • (NLS) N-Lauroylsarcosine sodium salt >=94% • Corning COSTAR 96-well clear flat-bottom assay plate • Wild-type yeast in 2x synthetic complete media at an OD600 of 0.1-0.2
3. Equipment: • Molecular Devices SpectraMax Plus 384 Microplate Reader
4.Procedure:
1. Make a 0.1% solution of NLS by putting 1µl of a 10% solution of NLS in 99 mL of water. This is going to give you a 100 mL solution with a 0.1 concentration of NLS. 2. Use the 0.1% solution of NLS to make 7 different concentrations of N-Lauroylsarcosine (0%, 0.001%, 0.002%, 0.005%, 0.01%, 0.02%, 0.05%). To increase solubility, temperature can be increased to 25-30°C. 3. Set up 8 wells according to following table.
| Well A (0%) | Well B (0.001%) | Well C (0.002%) | Well D (0.005%) | Well E (0.01%) | Well F (0.02%) | Well G (0.05%) | Well H (0.1%) | |
| Yeast culture | 50µl | 50µl | 50µl | 50µl | 50µl | 50µl | 50µl | 50µl |
| 0.1% solution of NLS | X | 0.5µl | 1.0µl | 2.5µl | 5.0µl | 10µl | 25µl | 50µl |
| Deionized water | 50µl | 49.5µl | 49µl | 47.5µl | 45µl | 40µl | 25µl | X |
4. Set up the plate reader as follows:
Temperature: 30°C Mode: Kinetic Wavelength: 600 nm Interval: 5 minutes Total run time: 24 hours Shake before read: 30 seconds
5. Transfer the assay plate to the reader and read for 24 hours. Notes • Results:
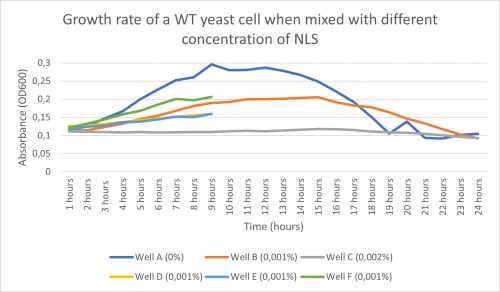
-Here is the graph obtained after following the procedure above and putting it in the plate reader for 24 hours:
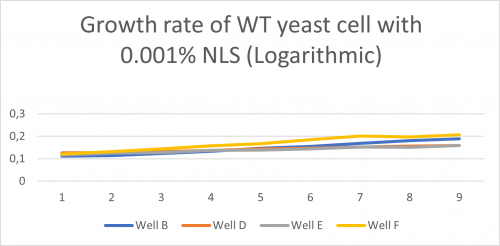
-Under is a graph with a Y axis scale set to logarithmic so we can find the doubling-time of the WT yeast cell in well B,D,E,F when mixed with a 0.001% solution of NLS:
Doubling time of Well B, D, E, F
-Td (Well B)= 547 minutes -Td (Well D) = 1442 minutes -Td (Well E) = 1086 minutes -Td (Well F) = 496 minutes -Td (average) = 893 minutes
Doubling Time for Well A (0% NLS) and Well C (0.002% NLS):
-Td (Well A) = 302 minutes -Td (Well C) = 10 570 minutes
• Analysis and Conclusions:
-According to the graph above and the doubling time found, we noticed that in well A (with only water), the growth of the WT yeast cell was significant with a doubling time of 302 minutes. In Well C (0,002% NLS), practically no growth occurred with having a doubling time of 10 570 minutes. Following this information, we concluded that 0,002% NLS was too concentrated for any growth to occur.
-Furthermore, after seeing that well B’s growth rate graph and its doubling time (893 minutes) were situated in between A’s and C’s, we concluded that 0,001% was the concentration we were going to use.
-Important to note: the concentration used need to be divided by 2 because out of the 100µl solution, 50µl is yeast cell so it dilutes the previous dilution. With that being said, we are technically using 0,0005% NLS.
Experiment 2:
NLS effect on KO yeast cells growth
1. Introduction: In this experiment, we will be testing the effect of N-Lauroylsarcosine on the speed of growth of different knocked-out yeast cells. Our objective is to find out if there is any knocked-out genes that are related to the growth of yeast cells.
2. Materials: • 0,001% (NLS) N-Lauroylsarcosine sodium salt >=94% • Corning COSTAR 96-well clear flat-bottom assay plate • 9 different Knocked-out yeast cells
3. Equipment: • Molecular Devices SpectraMax Plus 384 Microplate Reader
4. Procedure: 1. Make a 0,1% solution of NLS by putting 1µl of a 10% NLS solution in 99µl of DI water. 2. Mix the 0,1% solution with enough water to dilute the concentration to 0.001% while having a 50µl solution. Then, mix it with 9 different KO yeast cell to make nine different 100µl solution. 3. Set up 9 wells according to following table. Do two experiments of this.
| Well 1 (YDL109C) | Well 2 (YGL146C) | Well 3 (YOR111W) | Well 4 (YHL029C) | Well 5 (YDR307W) | Well 6 (YNL058C) | Well 7 (YCL049C) | Well 8 (YGR079W) | Well 9 (YBL113C) | |
| KO yeast cell | 50µl | 50µl | 50µl | 50µl | 50µl | 50µl | 50µl | 50µl | 50µl |
| 0.1% solution of NLS | 0.5µl | 0.5µl | 0.5µl | 0.5µl | 0.5µl | 0.5µl | 0.5µl | 0.5µl | 0.5µl |
| Deionized Water | 49.5 µl | 49.5 µl | 49.5 µl | 49.5 µl | 49.5 µl | 49.5 µl | 49.5 µl | 49.5 µl | 49.5 µl |
4. Set up the plate reader as follows:
Temperature: 30°C Mode: Kinetic Wavelength: 600 nm Interval: 5 minutes Total run time: 24 hours Shake before read: 30 seconds
5. Transfer the assay plate to the reader and read for 24 hours.
6. Collect the data and create a graph to compare results.