Difference between revisions of "UW-Stout/Bud Scars FA22"
| (9 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | + | '''Introduction''' | |
| + | When a yeast cell produces the bud of a daughter cell and the daughter cell splits off from the mother cell it creates a circular scar that can be seen under a microscope with fluorescence dye. Counting these scars can let us know how many offspring each cell has had. It can also give us an estimation of how old the cells are based on how many scars they have. The older they are the more scars that they will have. | ||
'''Materials''' | '''Materials''' | ||
| Line 5: | Line 6: | ||
*Knockout yeast culture | *Knockout yeast culture | ||
*1.7ml microcentrifuge tube (1 for each culture) | *1.7ml microcentrifuge tube (1 for each culture) | ||
| − | *Calcofluor White Stain Kit Reagent B- Calcofluor White (1.0g), Evans Blue Dye (0.4g), and demineralized Water (1000.0ml) (10 ul for each culture) | + | *Calcofluor White Stain Kit Reagent B- Calcofluor White (1.0g), Evans Blue Dye (0.4g), and demineralized Water (1000.0ml), (10 ul for each culture) |
*Glass Microscope Slide | *Glass Microscope Slide | ||
*Coverslip | *Coverslip | ||
*Microscope | *Microscope | ||
| − | *Distilled Water () | + | *Distilled Water (3,050 ul) |
'''Procedure''' | '''Procedure''' | ||
| Line 20: | Line 21: | ||
#Pellet, remove liquid and suspend in 10 ul of calcofluor | #Pellet, remove liquid and suspend in 10 ul of calcofluor | ||
#Incubate at room temperature for 5 minutes | #Incubate at room temperature for 5 minutes | ||
| − | #Centrifuge (13k for 60 seconds) remove liquid | + | #Centrifuge (13k for 60 seconds) and then remove liquid |
#Add 1 ml of water, vortex briefly, centrifuge (13k for 60 seconds), remove liquid | #Add 1 ml of water, vortex briefly, centrifuge (13k for 60 seconds), remove liquid | ||
#Resuspend pellet in 50 ul of water | #Resuspend pellet in 50 ul of water | ||
| Line 26: | Line 27: | ||
#Put a paper towel below and above and cover with a heavy object for 10 minutes | #Put a paper towel below and above and cover with a heavy object for 10 minutes | ||
#Look at the cells under a microscope and observe the number of bud scars on each cell. View 100 different cells | #Look at the cells under a microscope and observe the number of bud scars on each cell. View 100 different cells | ||
| + | |||
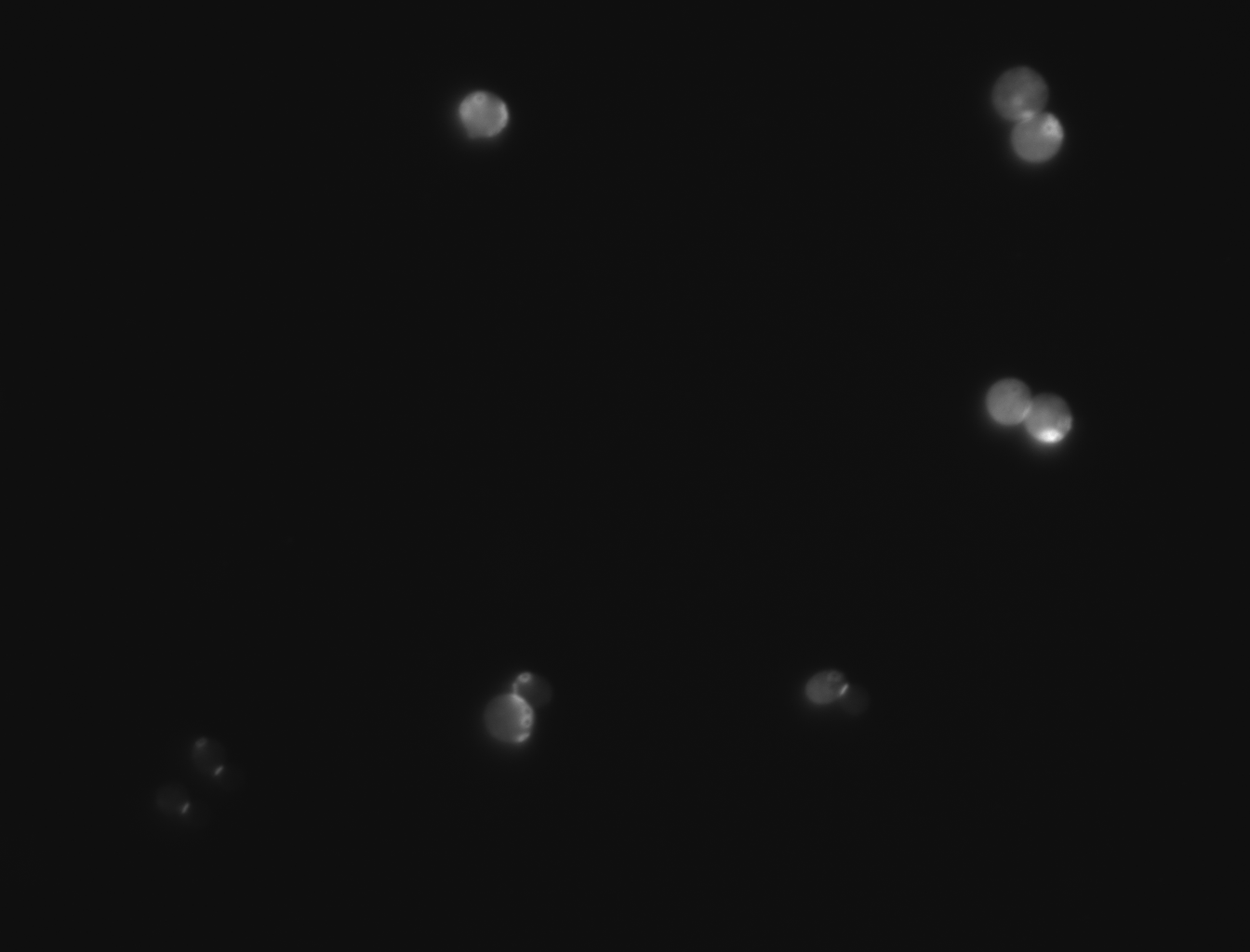
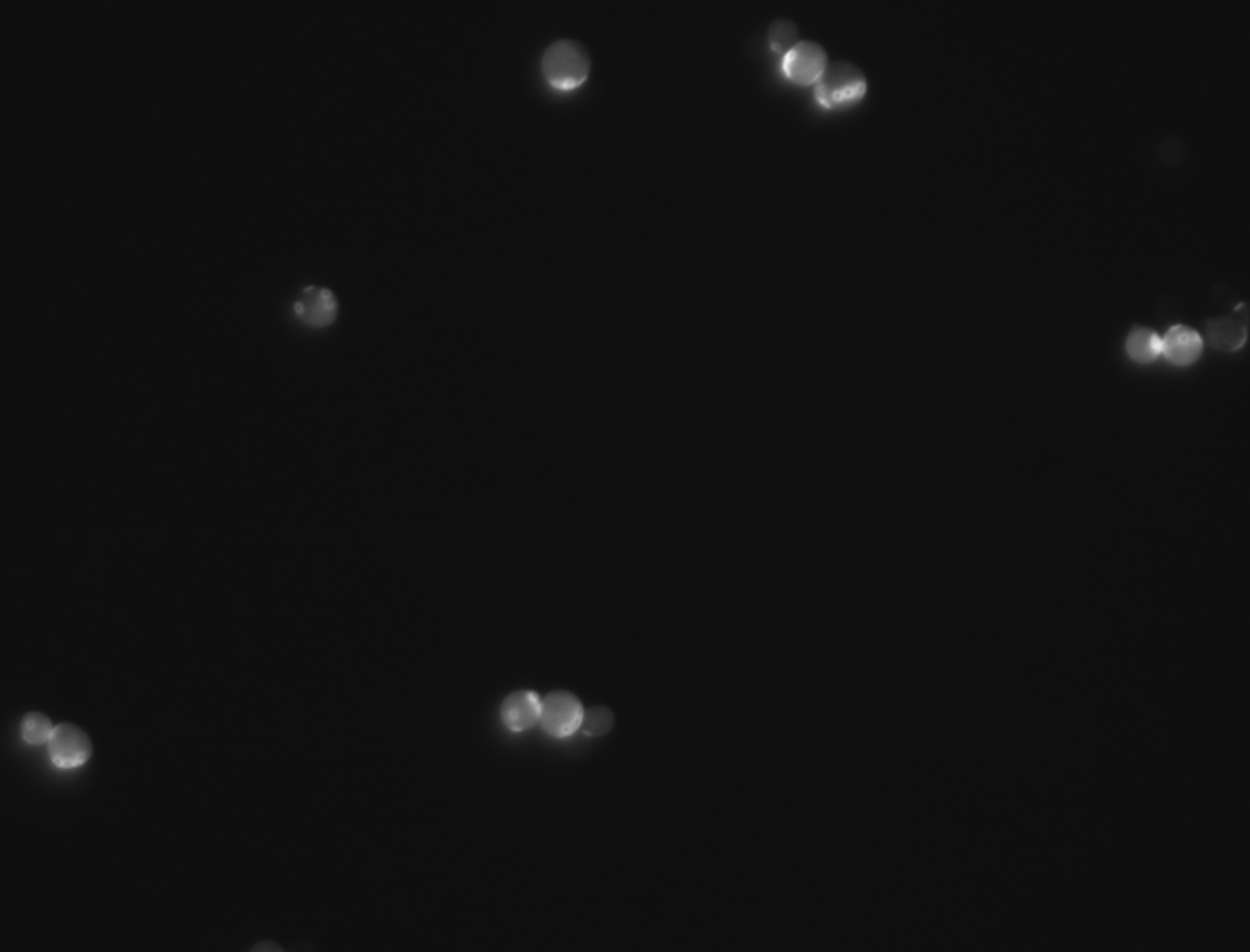
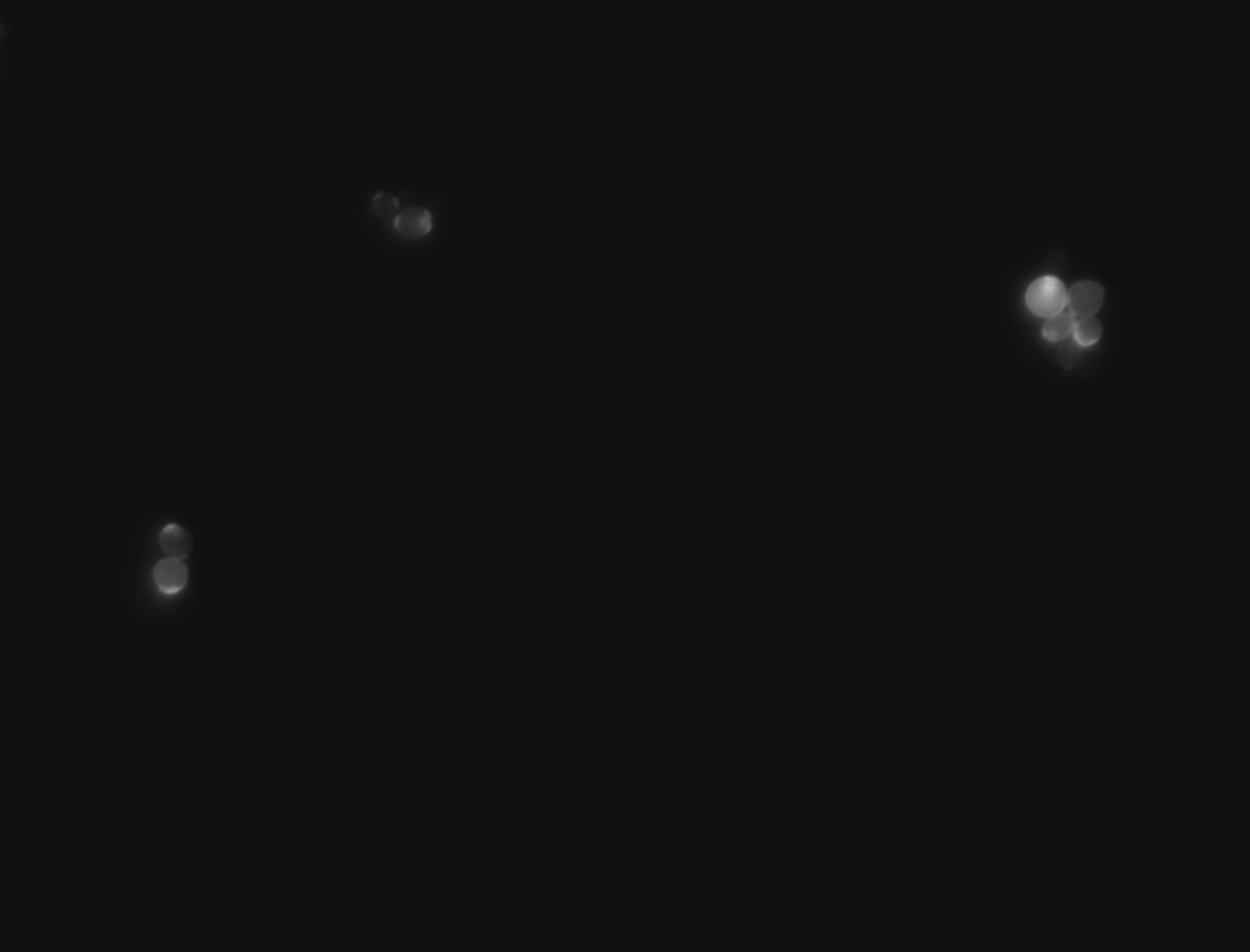
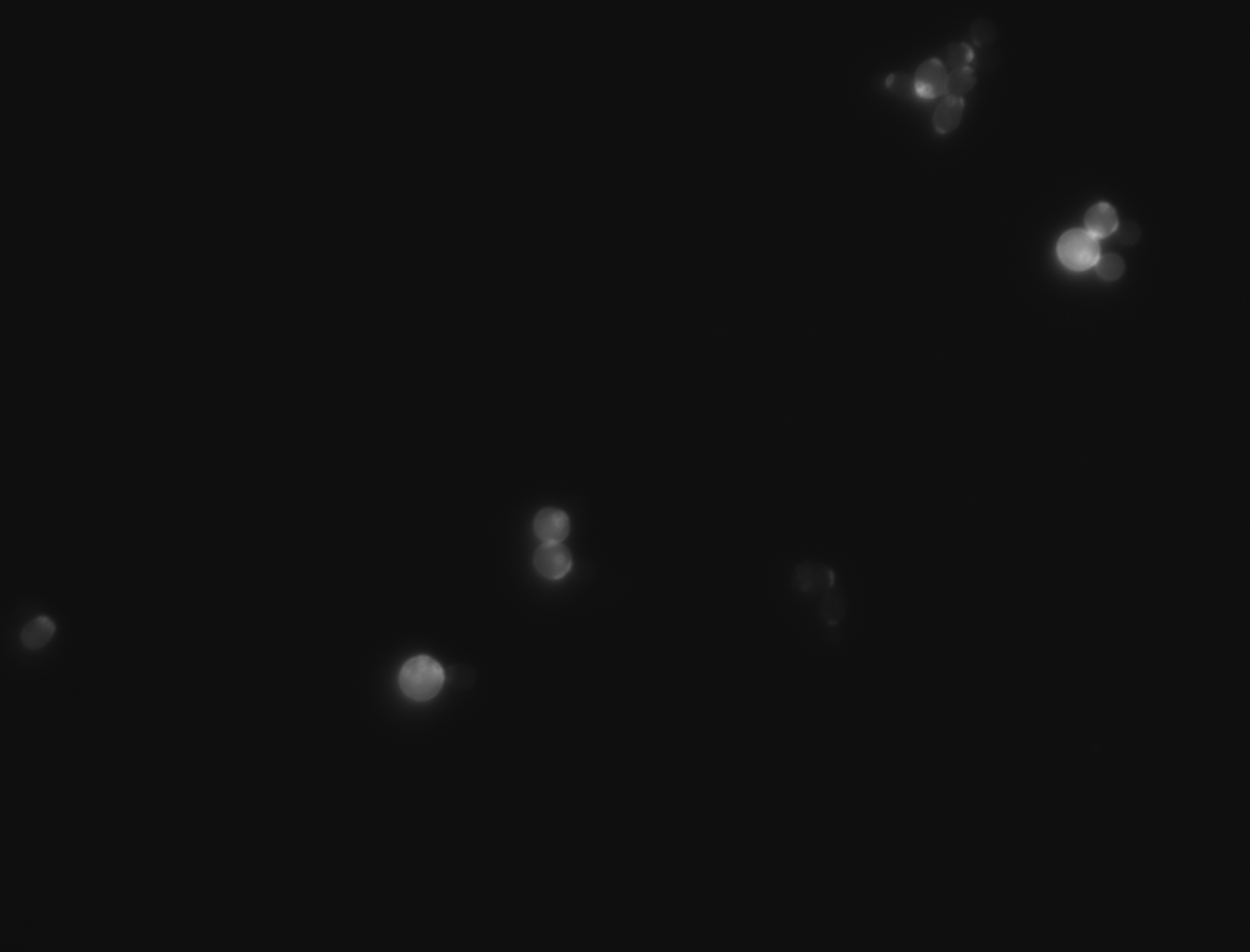
| + | -For this experiment we saved pictures of the yeast and counted the yeast until we had counted 100 cells and we recorded the amount of bud scars present. We had two people count the cells and if the numbers matched then we recorded that data and if the numbers did not match then we recounted the cells. No cells that were hard to see or oddly shaped were counted due to them possibly adding inaccuracies to the data | ||
| + | |||
| + | -We ended up getting an average of 2.46 bud scars per cell with the wild yeast strain (BY4735). | ||
| + | [[File:Yeast_wild(2)_1_9.png]] | ||
| + | [[File:Yeast_wild(2)_2_9.png]] | ||
| + | [[File:Yeast_wild(2)_3_11.png]] | ||
| + | [[File:Yeast_wild(2)_4_6.png]] | ||
| + | [[File:Yeast_wild(2)_5_13.png]] | ||
| + | [[File:Yeast_wild(2)_6_7.png]] | ||
| + | [[File:Yeast_wild(2)_7_15.png]] | ||
| + | [[File:Yeast_wild(2)_8_7.png]] | ||
| + | [[File:Yeast_wild(2)_9_9.png]] | ||
| + | [[File:Yeast_wild(2)_10_7.png]] | ||
| + | [[File:Yeast_wild(2)_11_13.png]] | ||
Latest revision as of 18:15, 20 December 2022
Introduction When a yeast cell produces the bud of a daughter cell and the daughter cell splits off from the mother cell it creates a circular scar that can be seen under a microscope with fluorescence dye. Counting these scars can let us know how many offspring each cell has had. It can also give us an estimation of how old the cells are based on how many scars they have. The older they are the more scars that they will have.
Materials
- Wild type yeast culture
- Knockout yeast culture
- 1.7ml microcentrifuge tube (1 for each culture)
- Calcofluor White Stain Kit Reagent B- Calcofluor White (1.0g), Evans Blue Dye (0.4g), and demineralized Water (1000.0ml), (10 ul for each culture)
- Glass Microscope Slide
- Coverslip
- Microscope
- Distilled Water (3,050 ul)
Procedure
- Gather materials
- Preserve cellular architecture and the composition of cells by adding 3.7% of formaldehyde and yeast cells to equal 1 ml of liquid
- Vortex briefly and let sit for 50 minutes
- Centrifuge (13k for 60 seconds) to make a pellet and remove liquid without disrupting the pellet
- Add 1 ml of water, vortex briefly, centrifuge (13k for 60 seconds), and remove liquid without disrupting the pellet
- Repeat steps 4 and 5
- Pellet, remove liquid and suspend in 10 ul of calcofluor
- Incubate at room temperature for 5 minutes
- Centrifuge (13k for 60 seconds) and then remove liquid
- Add 1 ml of water, vortex briefly, centrifuge (13k for 60 seconds), remove liquid
- Resuspend pellet in 50 ul of water
- Mount on the slide (5ul) and cover with a coverslip using petroleum jelly around the edges of the coverslip to prevent loss of moisture
- Put a paper towel below and above and cover with a heavy object for 10 minutes
- Look at the cells under a microscope and observe the number of bud scars on each cell. View 100 different cells
-For this experiment we saved pictures of the yeast and counted the yeast until we had counted 100 cells and we recorded the amount of bud scars present. We had two people count the cells and if the numbers matched then we recorded that data and if the numbers did not match then we recounted the cells. No cells that were hard to see or oddly shaped were counted due to them possibly adding inaccuracies to the data
-We ended up getting an average of 2.46 bud scars per cell with the wild yeast strain (BY4735).