Difference between revisions of "UW-Stout/pH Acid SP22"
| (21 intermediate revisions by the same user not shown) | |||
| Line 46: | Line 46: | ||
| − | [[Image:pilotgraph.jpg]] | + | [[Image:pilotgraph.jpg|900px]] |
| − | + | *remember OD600 is the "optical density" of the yeast | |
| − | ==Final Experiment | + | ==Final Experiment Protocol== |
'''Addition of Buffer to Yeast''' | '''Addition of Buffer to Yeast''' | ||
| Line 65: | Line 65: | ||
*Transfer the assay plate to the reader and run for 24 hours | *Transfer the assay plate to the reader and run for 24 hours | ||
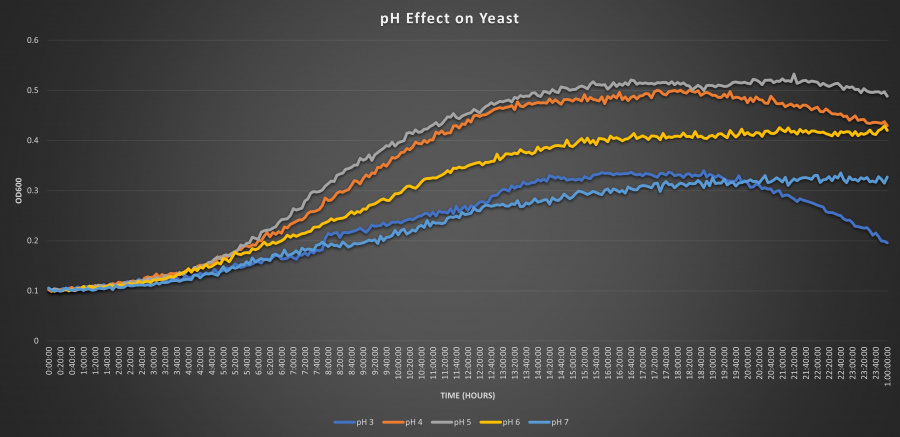
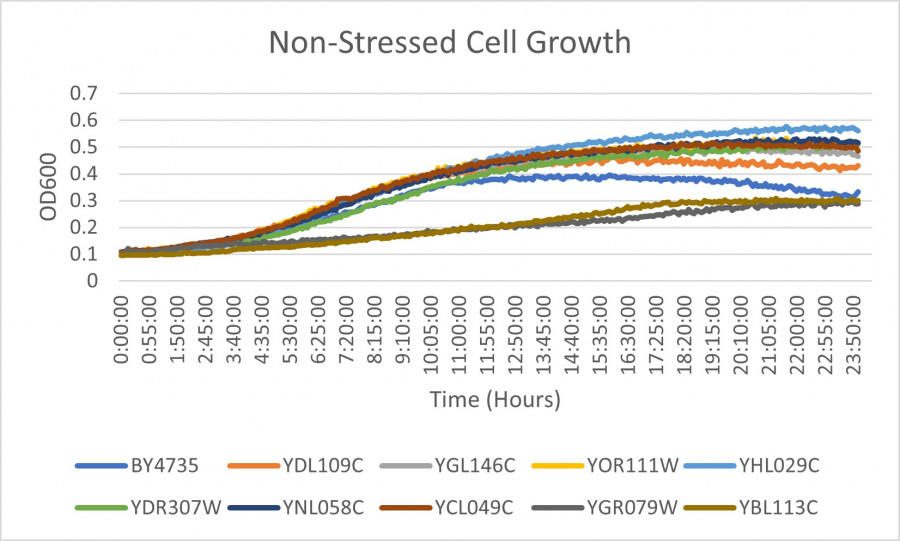
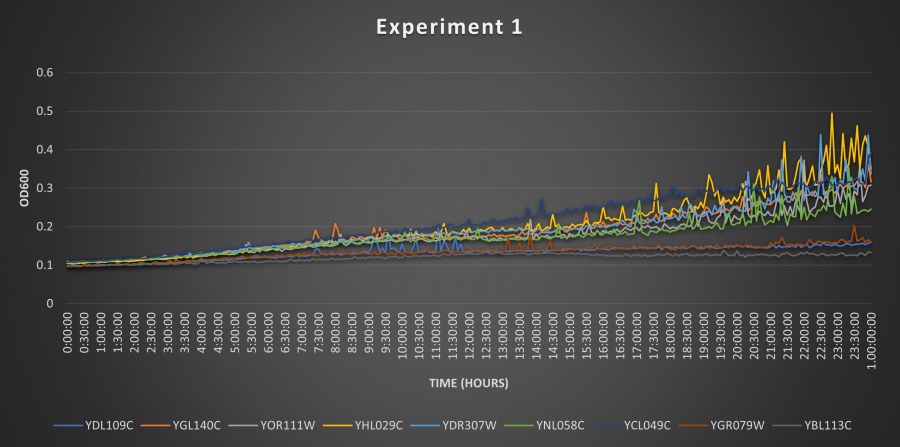
| − | ==Results | + | ==Results== |
| + | |||
| + | [[Image:nonstressed.jpg|900px]] | ||
| + | |||
| + | |||
| + | [[Image:EX2.jpg|900px]] | ||
| + | |||
| + | [[Image:EX1.jpg|900px]] | ||
| + | |||
| + | [[Image:G3.jpg|900px]] | ||
| + | |||
| + | |||
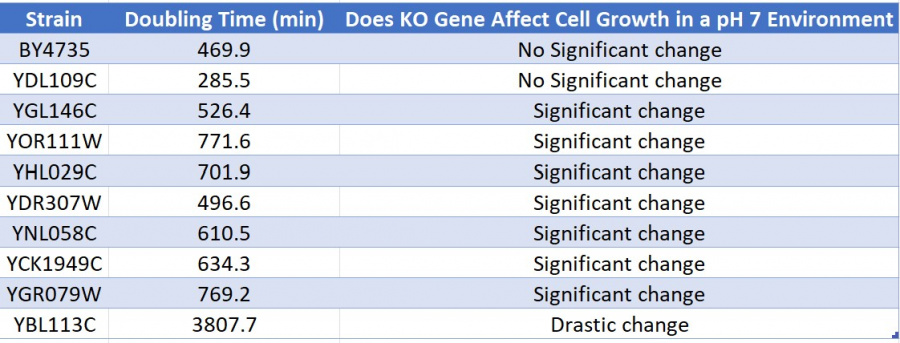
| + | ==Interpretation and Doubling Time== | ||
| + | |||
| + | [[Image:results.jpg|900px]] | ||
Latest revision as of 12:34, 3 May 2022
Contents
Introduction
Our protocol is designed to determine if the knocked-out gene affects or does not affect the growth of our yeasts in environments of pH 7. The pH of 7 was predetermined in a pilot experiment to be a pH that would stress the cell’s growth without killing all the cells. A citric acid monohydrate buffer was used to maintain a pH of 7 in the growth environments throughout the entire growth process.
Safety
Inhalation of disodium phosphate can lead to a cough, shortness of breath, and a sore throat. Getting disodium phosphate on your skin can lead to a rash and swelling. Getting it into your eyes can lead to redness and swelling also. Inhalation of citric acid monohydrate can lead to erosion of the teeth2. A fume hood, safety glasses and goggles, gloves and a lab coat will be required to handle this substance safely.
Materials/Equipment
- Citric Acid Monohydrate
- Disodium Phosphate
- De-ionized Water
- Micropipette
- 15ml tube (3x)
- 96-well clear flat-bottom assay plate
- Micropipette tips
- Gloves
- Incubator set to 30 C
- Scale
- Sterile cabinet
- pH test strips
- Yeast strains
Procedure
Preparing Buffer Stock Solutions (0.1M citric acid, 0.2M disodium phosphate)
- To obtain 0.1M of citric acid monohydrate, combine .21g of in 10ml of de-ionized water
- To obtain 0.2M of disodium phosphate, combine .28g in 10 ml de-ionized water
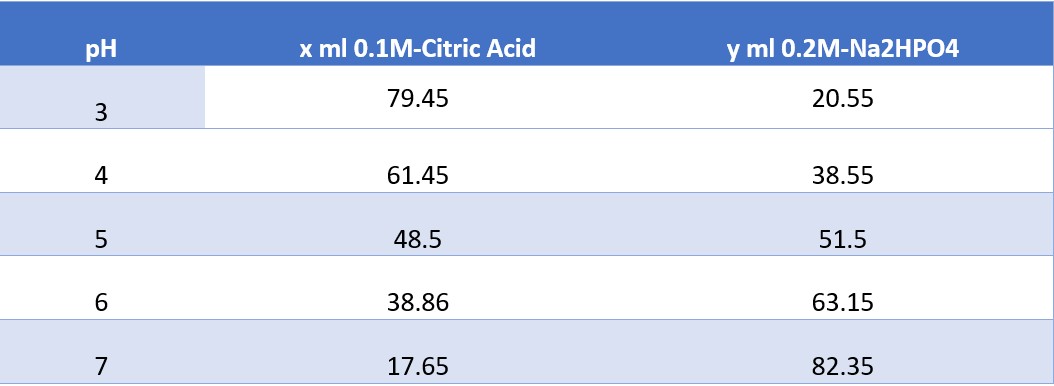
Preparing Buffer Solution
To create a buffer solution at a specific pH, you must combine x ml of citric acid monohydrate with y ml of disodium phosphate and shake well
pH x ml 0.1M-Citric Acid y ml 0.2M-Na2HPO4
Citric Acid Monohydrate-Disodium Phosphate Buffer Calibration Experiment
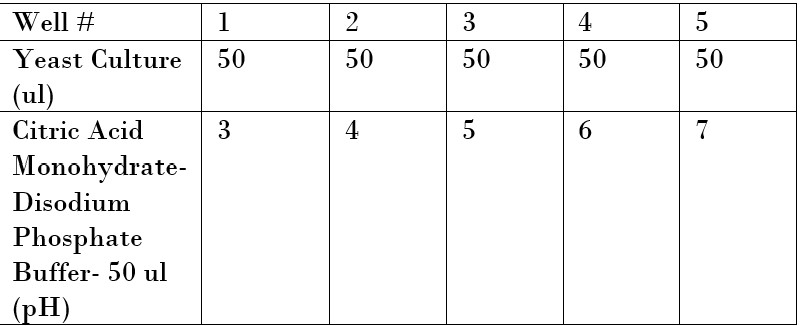
In this experiment, the pH of Citric Acid Monohydrate-Disodium Phosphate Buffer needs to have an effect on the yeast cells that is not too aggressive towards the yeast cells but affects them to show a difference.
Plating
- Determine the “sweet spot” solution
- Vortex yeast cells to resuspend the yeast cells briefly
- Various pHs of 3 pH, 4 pH,5 pH,6 pH, and 7 pH,10 pH, 10.2 pH were tested in this experiment. A total volume will always be 50 ul of the Citric Acid Monohydrate-Disodium Phosphate buffer in this experiment. The table shows the pH of Citric Acid Monohydrate-Disodium Phosphate buffer in each well. All plates will be a total volume of 100 ul.
- remember OD600 is the "optical density" of the yeast
Final Experiment Protocol
Addition of Buffer to Yeast
- Vortex wild type strain and knock out strains
- Add 50ul of each knock out strain to own each well
- Add 50ul of pH buffer to each subsequent well
Preparing Plate Reader
- Temperature: at 30 degrees Celsius
- Mode: Kinetic
- Read: 600 n
- Intervals: 5 minutes
- Total run time: 24 hours
- Shake before read: 30 seconds
- Transfer the assay plate to the reader and run for 24 hours
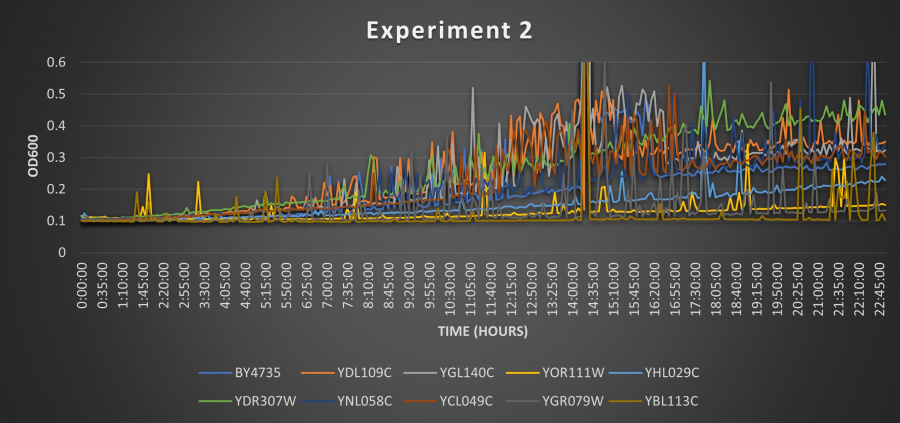
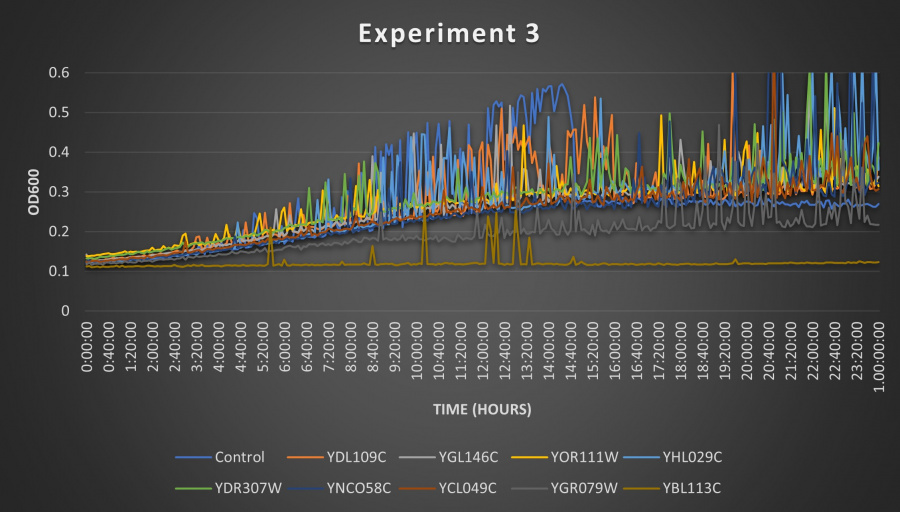
Results