Difference between revisions of "UW-Stout/Saline SP23"
| Line 3: | Line 3: | ||
== '''Pilot Experiment''' == | == '''Pilot Experiment''' == | ||
| − | == | + | #== Introduction == |
This experiment is testing how much salt will stress out the yeast without killing them. Our goal of this experiment is to see how much salt is needed to allow the yeast to grow while altering their growth curve without killing them. In this pilot experiment we tested wild yeast strains against different molarities of salt water. Looking at the growth curve data will help us determine which molarity of salt water stressed out the yeast without killing them. | This experiment is testing how much salt will stress out the yeast without killing them. Our goal of this experiment is to see how much salt is needed to allow the yeast to grow while altering their growth curve without killing them. In this pilot experiment we tested wild yeast strains against different molarities of salt water. Looking at the growth curve data will help us determine which molarity of salt water stressed out the yeast without killing them. | ||
| − | == Materials == | + | ##== Materials == |
*H2O | *H2O | ||
*NaCl (table salt) | *NaCl (table salt) | ||
*12 2 ml centrifuge tubes | *12 2 ml centrifuge tubes | ||
*Yeast cells | *Yeast cells | ||
| − | == | + | #== Procedure == |
#Using the calculation table that is provided, calculate the dilution from 4 M saltwater into the varying amounts.([https://word-edit.officeapps.live.com/we/WordViewer/request.pdf?WOPIsrc=https%3A%2F%2Fliveuwstout%2Dmy%2Esharepoint%2Ecom%2Fpersonal%2Frobertse3001%5Fmy%5Fuwstout%5Fedu%2F%5Fvti%5Fbin%2Fwopi%2Eashx%2Ffiles%2F8c1d9eaa693e4e718d34a8e4cc7861e4&access_token=eyJ0eXAiOiJKV1QiLCJhbGciOiJSUzI1NiIsIng1dCI6IkNRQU5lRWUtSUxVNTdlSnRZS0N2QVh2b1RkNCJ9.eyJhdWQiOiJ3b3BpL2xpdmV1d3N0b3V0LW15LnNoYXJlcG9pbnQuY29tQGI3MWE4MWEzLTJmOTUtNDM4MS05Yjg5LWM2MjM0M2E2NjA1MiIsImlzcyI6IjAwMDAwMDAzLTAwMDAtMGZmMS1jZTAwLTAwMDAwMDAwMDAwMEA5MDE0MDEyMi04NTE2LTExZTEtOGVmZi00OTMwNDkyNDAxOWIiLCJuYmYiOiIxNjgzNDE2ODQ2IiwiZXhwIjoiMTY4MzQ1Mjg0NiIsIm5hbWVpZCI6IjAjLmZ8bWVtYmVyc2hpcHxyb2JlcnRzZTMwMDFAbXkudXdzdG91dC5lZHUiLCJuaWkiOiJtaWNyb3NvZnQuc2hhcmVwb2ludCIsImlzdXNlciI6InRydWUiLCJjYWNoZWtleSI6IjBoLmZ8bWVtYmVyc2hpcHwxMDAzMjAwMTllMTFlMTljQGxpdmUuY29tIiwic2hhcmluZ2lkIjoiOTk0YjNhMjEtMjYwYy00ZWI5LWE0OWEtNWM5NjdhMGIzMjJkIiwic2lnbmluX3N0YXRlIjoiW1wia21zaVwiLFwiZHZjX2NtcFwiXSIsInV0aSI6IlJsbHNZUGlzNDBpWV9PMXFnblZJQUEiLCJpc2xvb3BiYWNrIjoiVHJ1ZSIsImFwcGN0eCI6IjhjMWQ5ZWFhNjkzZTRlNzE4ZDM0YThlNGNjNzg2MWU0O3ZYam1GZUVqbHNqeE0xWnkzMU9mRVZpSkFFMD07RGVmYXVsdDs7N0ZGRkZGRkZGRkZGRkZGRjtUcnVlOzs7MTA0ODY0NDszNjVhYjBhMC02MDYwLTMwMDAtOGU0Zi0wNTdjNDk0NzlhZmIiLCJmaWQiOiIxOTI5NjUifQ.dVxwv_0zx2Sxo31QbJxRZHhQBYTadHamgTzitKBqK7XEFgZFxXojwAmlWFFmY2DLP1fpthWE-MV2I2VT6uU9wnL6qN30U5UqSwHA1n5FiwMI2YxawseBMY3t72Z7pSYmzmXV7qZsOzefc7-ihcHbyef2h_KywR0-mvJSPM6XEwu5W6fNlHjMIXHDX93hSQb1FW7aahrrDS6j_wy9JPSEjavSnDi05Np8SOSqzA4e5cw7_DUsyYdEgYARPHP_vYdDTUcN2R5dvdrAu_z2A8Z_PI_JWGuPJZvFrr3TbJ-hQiegcO3X5CXleegozpdyz5QzL0MZ6xVAWWLY5WTyFe9neA&access_token_ttl=1683452849737&type=downloadpdf&rndm=a40d1917-ce50-465c-b734-a1a75c4f0e20&usid=778a8f4f-3b6d-4079-a331-cfa2345b9fdd&build=16.0.16502.41004&waccluster=PUS3 concentration table]) | #Using the calculation table that is provided, calculate the dilution from 4 M saltwater into the varying amounts.([https://word-edit.officeapps.live.com/we/WordViewer/request.pdf?WOPIsrc=https%3A%2F%2Fliveuwstout%2Dmy%2Esharepoint%2Ecom%2Fpersonal%2Frobertse3001%5Fmy%5Fuwstout%5Fedu%2F%5Fvti%5Fbin%2Fwopi%2Eashx%2Ffiles%2F8c1d9eaa693e4e718d34a8e4cc7861e4&access_token=eyJ0eXAiOiJKV1QiLCJhbGciOiJSUzI1NiIsIng1dCI6IkNRQU5lRWUtSUxVNTdlSnRZS0N2QVh2b1RkNCJ9.eyJhdWQiOiJ3b3BpL2xpdmV1d3N0b3V0LW15LnNoYXJlcG9pbnQuY29tQGI3MWE4MWEzLTJmOTUtNDM4MS05Yjg5LWM2MjM0M2E2NjA1MiIsImlzcyI6IjAwMDAwMDAzLTAwMDAtMGZmMS1jZTAwLTAwMDAwMDAwMDAwMEA5MDE0MDEyMi04NTE2LTExZTEtOGVmZi00OTMwNDkyNDAxOWIiLCJuYmYiOiIxNjgzNDE2ODQ2IiwiZXhwIjoiMTY4MzQ1Mjg0NiIsIm5hbWVpZCI6IjAjLmZ8bWVtYmVyc2hpcHxyb2JlcnRzZTMwMDFAbXkudXdzdG91dC5lZHUiLCJuaWkiOiJtaWNyb3NvZnQuc2hhcmVwb2ludCIsImlzdXNlciI6InRydWUiLCJjYWNoZWtleSI6IjBoLmZ8bWVtYmVyc2hpcHwxMDAzMjAwMTllMTFlMTljQGxpdmUuY29tIiwic2hhcmluZ2lkIjoiOTk0YjNhMjEtMjYwYy00ZWI5LWE0OWEtNWM5NjdhMGIzMjJkIiwic2lnbmluX3N0YXRlIjoiW1wia21zaVwiLFwiZHZjX2NtcFwiXSIsInV0aSI6IlJsbHNZUGlzNDBpWV9PMXFnblZJQUEiLCJpc2xvb3BiYWNrIjoiVHJ1ZSIsImFwcGN0eCI6IjhjMWQ5ZWFhNjkzZTRlNzE4ZDM0YThlNGNjNzg2MWU0O3ZYam1GZUVqbHNqeE0xWnkzMU9mRVZpSkFFMD07RGVmYXVsdDs7N0ZGRkZGRkZGRkZGRkZGRjtUcnVlOzs7MTA0ODY0NDszNjVhYjBhMC02MDYwLTMwMDAtOGU0Zi0wNTdjNDk0NzlhZmIiLCJmaWQiOiIxOTI5NjUifQ.dVxwv_0zx2Sxo31QbJxRZHhQBYTadHamgTzitKBqK7XEFgZFxXojwAmlWFFmY2DLP1fpthWE-MV2I2VT6uU9wnL6qN30U5UqSwHA1n5FiwMI2YxawseBMY3t72Z7pSYmzmXV7qZsOzefc7-ihcHbyef2h_KywR0-mvJSPM6XEwu5W6fNlHjMIXHDX93hSQb1FW7aahrrDS6j_wy9JPSEjavSnDi05Np8SOSqzA4e5cw7_DUsyYdEgYARPHP_vYdDTUcN2R5dvdrAu_z2A8Z_PI_JWGuPJZvFrr3TbJ-hQiegcO3X5CXleegozpdyz5QzL0MZ6xVAWWLY5WTyFe9neA&access_token_ttl=1683452849737&type=downloadpdf&rndm=a40d1917-ce50-465c-b734-a1a75c4f0e20&usid=778a8f4f-3b6d-4079-a331-cfa2345b9fdd&build=16.0.16502.41004&waccluster=PUS3 concentration table]) | ||
#Make these solutions in each of the 12 2 ml centrifuge tubes | #Make these solutions in each of the 12 2 ml centrifuge tubes | ||
| Line 16: | Line 16: | ||
#Micropipette 50 ul of the differing saltwater solutions into each well | #Micropipette 50 ul of the differing saltwater solutions into each well | ||
#Put into plate reader for 24-48 hours | #Put into plate reader for 24-48 hours | ||
| − | == | + | #== Data== |
[[File:data_1-jpeg%22.jpeg#file]] | [[File:data_1-jpeg%22.jpeg#file]] | ||
| − | == | + | #== Results== |
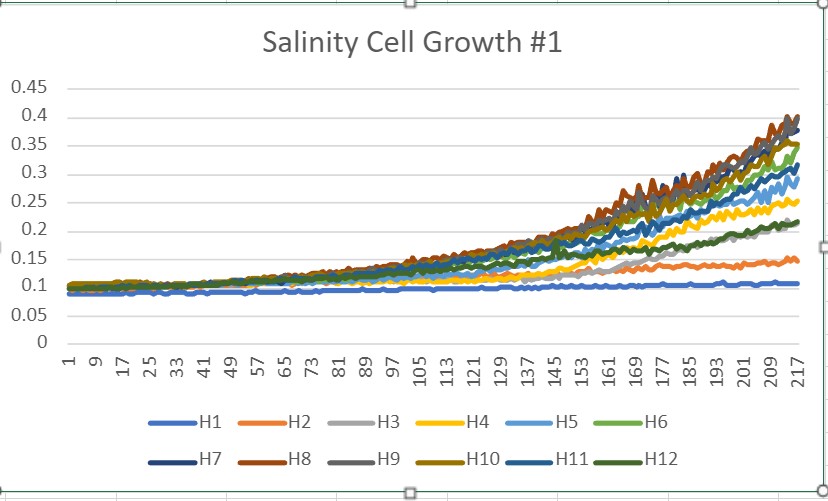
The graph above shows the growth of our yeast that was mixed with all the different salinity solutions. You can see in the graph that the low salinity solutions didn't really have an effect on the yeast growth. But all the higher salinity solutions had too much of an effect on the yeast. So, with that information, we decided to go with the 2 M concentration because it stressed out the yeast without killing them. | The graph above shows the growth of our yeast that was mixed with all the different salinity solutions. You can see in the graph that the low salinity solutions didn't really have an effect on the yeast growth. But all the higher salinity solutions had too much of an effect on the yeast. So, with that information, we decided to go with the 2 M concentration because it stressed out the yeast without killing them. | ||
Revision as of 17:32, 6 May 2023
Pilot Experiment
- == Introduction ==
This experiment is testing how much salt will stress out the yeast without killing them. Our goal of this experiment is to see how much salt is needed to allow the yeast to grow while altering their growth curve without killing them. In this pilot experiment we tested wild yeast strains against different molarities of salt water. Looking at the growth curve data will help us determine which molarity of salt water stressed out the yeast without killing them.
- == Materials ==
- H2O
- NaCl (table salt)
- 12 2 ml centrifuge tubes
- Yeast cells
- == Procedure ==
- Using the calculation table that is provided, calculate the dilution from 4 M saltwater into the varying amounts.(concentration table)
- Make these solutions in each of the 12 2 ml centrifuge tubes
- Micropipette 50 ul of yeast cells into 12 different wells
- Micropipette 50 ul of the differing saltwater solutions into each well
- Put into plate reader for 24-48 hours
- == Data==
- == Results==
The graph above shows the growth of our yeast that was mixed with all the different salinity solutions. You can see in the graph that the low salinity solutions didn't really have an effect on the yeast growth. But all the higher salinity solutions had too much of an effect on the yeast. So, with that information, we decided to go with the 2 M concentration because it stressed out the yeast without killing them.