Difference between revisions of "UW-Stout/Acids SP23"
(Pilot Experiment) |
(→Results) |
||
| (13 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | ==Introduction== | + | =='''Pilot Experiment'''== |
| + | ===Introduction=== | ||
We are looking to find a pH that puts yeast under stress. Our end goal is to be able to test different knockout yeast strains against an acidic medium, but to do this we first need to find a pH that allows the yeast to grow, but puts some strain on the growth curve. In this protocol, we are testing wild yeast strains against different concentration of acetic acid. By analyzing the growth curves, we will be able to find a concentration that will stress the yeast growth. | We are looking to find a pH that puts yeast under stress. Our end goal is to be able to test different knockout yeast strains against an acidic medium, but to do this we first need to find a pH that allows the yeast to grow, but puts some strain on the growth curve. In this protocol, we are testing wild yeast strains against different concentration of acetic acid. By analyzing the growth curves, we will be able to find a concentration that will stress the yeast growth. | ||
| − | ==Materials== | + | ===Materials=== |
| − | * | + | *Wild-type yeast in 2x synthetic complete media at an OD600 of 0.1-0.2 |
*17.4 M Glacial Acetic Acid | *17.4 M Glacial Acetic Acid | ||
*Deionized Water | *Deionized Water | ||
*Sterile Water | *Sterile Water | ||
*Pipettes | *Pipettes | ||
| − | * | + | *Corning COSTAR 96-well clear flat-bottom assay plate |
*1.7 mL microcentrifuge tubes | *1.7 mL microcentrifuge tubes | ||
*Disposable Gloves | *Disposable Gloves | ||
| − | ==Procedure== | + | ===Procedure=== |
#Using 17.4 M Glacial Acetic Acid, create a diluted stock solution | #Using 17.4 M Glacial Acetic Acid, create a diluted stock solution | ||
| − | + | #:*Use gloves when working with concentrated acid | |
| − | + | #:*Add 1.04 mL of Glacial Acetic Acid to 8.95 mL of Deionized Water | |
#Using this stock solution, create further dilutions | #Using this stock solution, create further dilutions | ||
| − | + | #:*In microcentrifuge tube, add 1 mL of Glacial Acetic Acid Dilution and label this tube 1 | |
| − | + | #:*Take 10 uL of solution out of tube one and place in another microcentrifuge tube, labeled 2 | |
| − | + | #:*Add 990 uL of sterile water to tube 2 | |
| − | + | #:*Using tube 2, repeat this process to create tube 3 | |
| − | + | #:*Repeat until there are 10 dilution tubes | |
| − | #Add 50 uL of BY yeast to wells 1-11 in the | + | #Add 50 uL of BY yeast to wells 1-11 in the assay plate |
#Add 50 uL of each dilution to the well sheet | #Add 50 uL of each dilution to the well sheet | ||
| − | + | #:*Add dilution 1 to well 1, dilution 2 to well 2, and so on | |
| − | + | #:*Use pipette tip to mix solutions | |
#Add 50 uL of sterile water to well 11 | #Add 50 uL of sterile water to well 11 | ||
| − | ::*Use pipette tip to mix | + | #:*Use pipette tip to mix solution |
| + | #Set up plate reader as follows: | ||
| + | #:*Temperature - 30 degrees Celsius | ||
| + | #:*Mode - Kinetic | ||
| + | #:*Wavelength - 600 nm | ||
| + | #:*Interval - 5 min | ||
| + | #:*Total Run Time - 24 hours | ||
| + | #:*Shake Before Read - 30 seconds | ||
| + | #Transfer assay plate to reader and read for 24 hours | ||
| + | ===Data=== | ||
| + | [[Image:AceticAcidGraph2.jpg]] | ||
| + | ===Results=== | ||
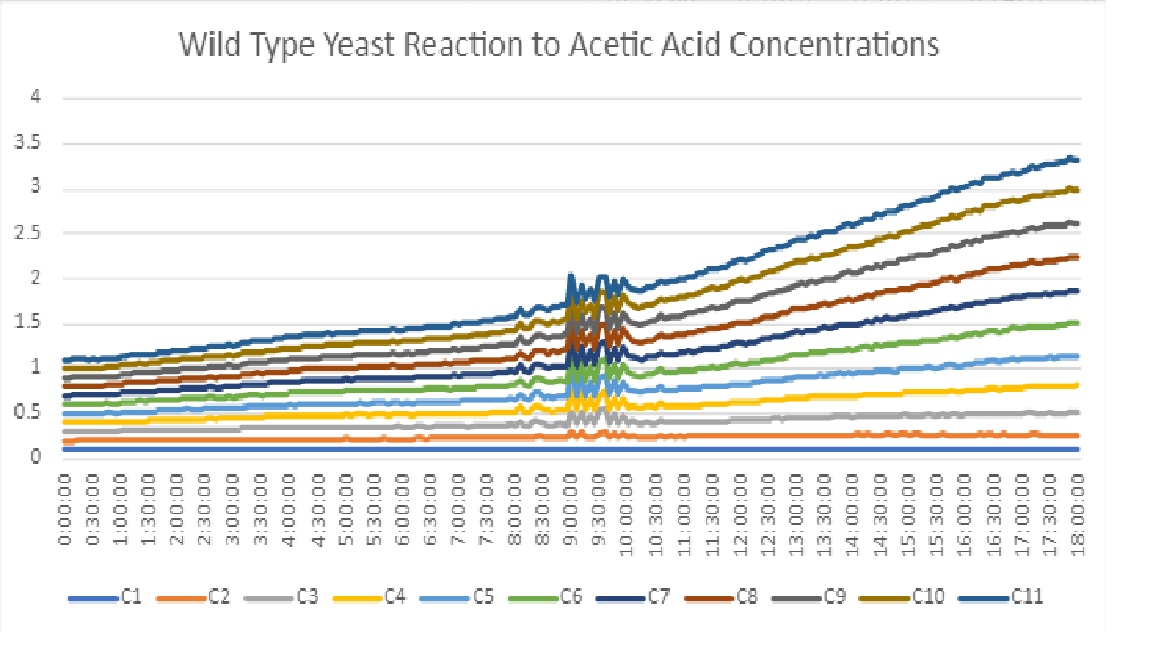
| + | Our results show that acid became more diluted, the yeast had a more successful growth curve. Since we were looking for an acid concentration that would stress yeast growth, but still allow there to be growth, we decided to go with a concentration in the middle. We settled on concentration 6 for our final experiment. | ||
| + | |||
| + | =='''Final Experiment'''== | ||
| + | ===Introduction=== | ||
| + | After determining an acetic acid concentration that would cause the yeast growth stress, we used this medium to test the reaction of different yeast strains. Each strain has a different gene knocked out, so by looking at its reaction to the acidic environment compared to the wild type control, we will be able to see if the gene that was knocked out had any function in the cells response to stress. | ||
| + | ===Materials=== | ||
| + | *Wild-type yeast in 2x synthetic complete media at an OD600 of 0.1-0.2 | ||
| + | *Knockout yeast strain YMR144W (labeled as 1) | ||
| + | *Knockout yeast strain SSK1 (labeled as 2) | ||
| + | *Knockout yeast strain YKL162C (labeled as 3) | ||
| + | *Pipettes | ||
| + | *Acetic acid concentration 6 | ||
| + | *Corning COSTAR 96-well clear flat-bottom assay plate | ||
| + | *Gloves | ||
| + | ===Procedure=== | ||
| + | #Obtain materials and gloves | ||
| + | #Using pipette, put 50 ul of acetic acid concentration 6 to wells 1-12 | ||
| + | #Using pipette, add 50 ul of yeast to acetic acid concentration 6 | ||
| + | #:*Add wild type yeast to wells 1-3 | ||
| + | #:*Add yeast strain 1 to wells 4-6 | ||
| + | #:*Add yeast strain 2 to wells 7-9 | ||
| + | #:*Add yeast strain 3 to wells 10-12 | ||
| + | #Use pipette tip to gently mix | ||
| + | #Set up plate reader as follows: | ||
| + | #:*Temperature - 30 degrees Celsius | ||
| + | #:*Mode - Kinetic | ||
| + | #:*Wavelength - 600 nm | ||
| + | #:*Interval - 5 min | ||
| + | #:*Total Run Time - 24 hours | ||
| + | #:*Shake Before Read - 30 seconds | ||
| + | #Transfer assay plate to reader and read for 24 hours | ||
Latest revision as of 10:51, 5 May 2023
Contents
Pilot Experiment
Introduction
We are looking to find a pH that puts yeast under stress. Our end goal is to be able to test different knockout yeast strains against an acidic medium, but to do this we first need to find a pH that allows the yeast to grow, but puts some strain on the growth curve. In this protocol, we are testing wild yeast strains against different concentration of acetic acid. By analyzing the growth curves, we will be able to find a concentration that will stress the yeast growth.
Materials
- Wild-type yeast in 2x synthetic complete media at an OD600 of 0.1-0.2
- 17.4 M Glacial Acetic Acid
- Deionized Water
- Sterile Water
- Pipettes
- Corning COSTAR 96-well clear flat-bottom assay plate
- 1.7 mL microcentrifuge tubes
- Disposable Gloves
Procedure
- Using 17.4 M Glacial Acetic Acid, create a diluted stock solution
- Use gloves when working with concentrated acid
- Add 1.04 mL of Glacial Acetic Acid to 8.95 mL of Deionized Water
- Using this stock solution, create further dilutions
- In microcentrifuge tube, add 1 mL of Glacial Acetic Acid Dilution and label this tube 1
- Take 10 uL of solution out of tube one and place in another microcentrifuge tube, labeled 2
- Add 990 uL of sterile water to tube 2
- Using tube 2, repeat this process to create tube 3
- Repeat until there are 10 dilution tubes
- Add 50 uL of BY yeast to wells 1-11 in the assay plate
- Add 50 uL of each dilution to the well sheet
- Add dilution 1 to well 1, dilution 2 to well 2, and so on
- Use pipette tip to mix solutions
- Add 50 uL of sterile water to well 11
- Use pipette tip to mix solution
- Set up plate reader as follows:
- Temperature - 30 degrees Celsius
- Mode - Kinetic
- Wavelength - 600 nm
- Interval - 5 min
- Total Run Time - 24 hours
- Shake Before Read - 30 seconds
- Transfer assay plate to reader and read for 24 hours
Data
Results
Our results show that acid became more diluted, the yeast had a more successful growth curve. Since we were looking for an acid concentration that would stress yeast growth, but still allow there to be growth, we decided to go with a concentration in the middle. We settled on concentration 6 for our final experiment.
Final Experiment
Introduction
After determining an acetic acid concentration that would cause the yeast growth stress, we used this medium to test the reaction of different yeast strains. Each strain has a different gene knocked out, so by looking at its reaction to the acidic environment compared to the wild type control, we will be able to see if the gene that was knocked out had any function in the cells response to stress.
Materials
- Wild-type yeast in 2x synthetic complete media at an OD600 of 0.1-0.2
- Knockout yeast strain YMR144W (labeled as 1)
- Knockout yeast strain SSK1 (labeled as 2)
- Knockout yeast strain YKL162C (labeled as 3)
- Pipettes
- Acetic acid concentration 6
- Corning COSTAR 96-well clear flat-bottom assay plate
- Gloves
Procedure
- Obtain materials and gloves
- Using pipette, put 50 ul of acetic acid concentration 6 to wells 1-12
- Using pipette, add 50 ul of yeast to acetic acid concentration 6
- Add wild type yeast to wells 1-3
- Add yeast strain 1 to wells 4-6
- Add yeast strain 2 to wells 7-9
- Add yeast strain 3 to wells 10-12
- Use pipette tip to gently mix
- Set up plate reader as follows:
- Temperature - 30 degrees Celsius
- Mode - Kinetic
- Wavelength - 600 nm
- Interval - 5 min
- Total Run Time - 24 hours
- Shake Before Read - 30 seconds
- Transfer assay plate to reader and read for 24 hours