Difference between revisions of "UW-Stout/Formamide SP22"
(→Procedure) |
(→Results) |
||
| (39 intermediate revisions by the same user not shown) | |||
| Line 5: | Line 5: | ||
===Materials / Equipment=== | ===Materials / Equipment=== | ||
| − | *Neat Formamide, 33ul | + | *Neat Formamide, '''33ul''' |
| − | *Sterile water, 567ul | + | *Sterile water, '''567ul''' |
| − | *0.2ml flat cap PCR tube, 13 | + | *0.2ml flat cap PCR tube, '''13 tubes''' |
| − | * | + | *Corning COSTAR 96-well clear flat-bottom assay plate |
| − | *Wild yeast | + | *Wild-type yeast in 2x synthetic complete media at an OD600 of 0.1-0.2, '''600ul''' |
*Molecular Devices SpectraMax Plus 384 Microplate Reader | *Molecular Devices SpectraMax Plus 384 Microplate Reader | ||
| − | *Micropipet 100ul | + | *Micropipet (100ul) |
| + | *Micropipet (10ul) | ||
| + | *Micropipet (2ul) | ||
*Micropipet tips | *Micropipet tips | ||
| Line 31: | Line 33: | ||
# Obtain the neat formamide (refrigerated) and sterile water | # Obtain the neat formamide (refrigerated) and sterile water | ||
# Obtain and label 12 PCR tubes 1-12 | # Obtain and label 12 PCR tubes 1-12 | ||
| − | # Obtain one more PCR tube to transfer 33ul of neat formamide inside, can put excess in | + | # Obtain one more PCR tube to transfer 33ul of neat formamide inside, can put excess formamide in |
| − | # | + | # Using the 13th PCR tube, pipet appropriate amount of formamide and sterile water into the labeled PCR tubes according to number |
| − | + | # In a sterile environment, pipet the solution (50ul) from the PCR tube into the well cell | |
| − | # In a sterile environment, pipet the | + | # Vortex the yeast culture briefly to resuspend the yeast cells, and then pipet 50ul of the wild yeast cells into each well in addition to the formamide-water solution |
| − | # | + | # Set up the plate reader as follows: |
| + | ## Temperature: 30 degrees Celsius | ||
| + | ## Mode: kinetic | ||
| + | ## Wavelength: 600 nm | ||
| + | ## Interval: 5 minutes | ||
| + | ## Total run time: 24 hours | ||
| + | ## Shake before read: 30 seconds | ||
| + | # Transfer the assay plate to the reader and read for 24 hours | ||
| + | # Record data | ||
===Results=== | ===Results=== | ||
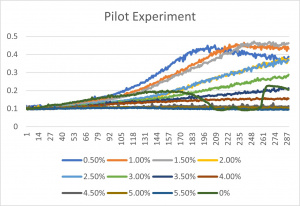
| − | [[File: | + | [[File:pilot1.jpg|300px|]] |
| + | * The x-axis is in time (min) and the y-axis is optical density at 600 | ||
| + | *The x-axis corresponds to the concentrations listed up above under the title "formamide concentrations" | ||
| + | *0% is an experimental error- since it has a concentration of 0% formamide anyway, it was left in the final graph | ||
| − | |||
| − | |||
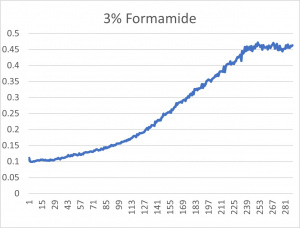
| + | [[File:3formamide.jpg|300px|]] | ||
| − | + | ||
| + | * The x-axis is in time (min) and the y-axis is optical density at 600 | ||
| + | *The 3% formamide used in the pilot experiment and represented as the light blue color (in the top-most picture) was chosen to be used on the transformed yeast cells | ||
| + | **This was deduced due to the position of the yeast cells under 3% formamide stress | ||
| + | ** Under 3% formamide, the yeast cells were able to grow, but didn't grow too much- simply put, the 3% line was right in the middle of the graph | ||
==Knockout Yeast Cell Gene== | ==Knockout Yeast Cell Gene== | ||
===Materials / Equipment=== | ===Materials / Equipment=== | ||
| − | *Neat formamide, 18ul | + | *Neat formamide, '''18ul''' |
| − | *Sterile water, 423ul | + | *Sterile water, '''423ul''' |
| − | *0.2ml flat cap PCR tube 10 | + | *0.2ml flat cap PCR tube, '''10 tubes''' |
*Well plate | *Well plate | ||
*Knock out yeast cells | *Knock out yeast cells | ||
*Molecular Devices SpectraMax Plus 384 Microplate Reader | *Molecular Devices SpectraMax Plus 384 Microplate Reader | ||
| − | *Micropipet 100ul | + | *Micropipet (100ul) |
| + | *Micropipet (20ul) | ||
| + | *Micropipet (2ul) | ||
*Micropipet tips | *Micropipet tips | ||
===Procedure=== | ===Procedure=== | ||
| − | # | + | # Obtain neat formamide (refrigerated) and sterile water |
| − | # | + | # Obtain and label PCR tubes 1-9 |
| − | # Pipet approximately 18ul formamide into a PCR tube, can put excess in | + | # Pipet approximately 18ul formamide into a 10th PCR tube, can put excess formamide in |
| − | # | + | # Using the 10th PCR tube, pipet 3.0ul of formamide into each PCR tube (labeled 1-9) |
# Pipet 47.0ul of sterile water into each of the numbered PCR tubes | # Pipet 47.0ul of sterile water into each of the numbered PCR tubes | ||
| − | # In a sterile environment, pipet 50ul of | + | # In a sterile environment, pipet 50ul of formamide-water solution from the PCR tubes into well cells |
| − | # | + | #Vortex each yeast culture strain briefly to resuspend the cells, and then pipet 50ul of each knockout strain into the appropriate well cells in addition to the solution |
| + | # Set up the plate reader as follows: | ||
| + | ## Temperature: 30 degrees Celsius | ||
| + | ## Mode: kinetic | ||
| + | ## Wavelength: 600 nm | ||
| + | ## Interval: 5 minutes | ||
| + | ## Total run time: 24 hours | ||
| + | ## Shake before read: 30 seconds | ||
| + | # Transfer the assay plate to the reader and read for 24 hours | ||
| + | # Record data | ||
===Results=== | ===Results=== | ||
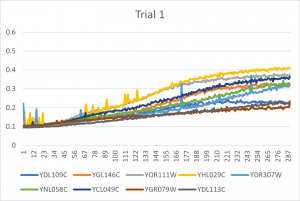
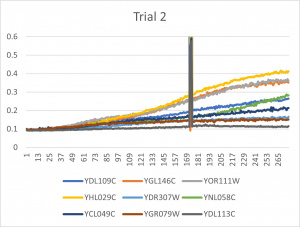
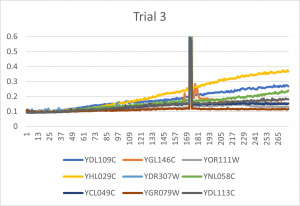
| + | [[File:trial11.jpg|300px|]] [[File:trial22.jpg|300px|]] [[File:trial3.jpg|300px|]] | ||
| + | |||
| + | * The x-axis is in time (min) and the y-axis is optical density at 600 | ||
| + | *Ran three trials, trial one through three is posted up above in picture form as a line graph | ||
| + | *The dark line in the middle of trail 2 and 3 is a scanning error from the Molecular Devices SpectraMax Plus 384 Microplate Reader | ||
| + | *Computed doubling times from the average times of the three trials for further analyzation | ||
Latest revision as of 19:49, 9 May 2022
Contents
Wild Yeast Cell Pilot Procedure
Caution
Neat Formamide is harmful to the eyes, if swallowed, inhaled, or absorbed through the skin.
Materials / Equipment
- Neat Formamide, 33ul
- Sterile water, 567ul
- 0.2ml flat cap PCR tube, 13 tubes
- Corning COSTAR 96-well clear flat-bottom assay plate
- Wild-type yeast in 2x synthetic complete media at an OD600 of 0.1-0.2, 600ul
- Molecular Devices SpectraMax Plus 384 Microplate Reader
- Micropipet (100ul)
- Micropipet (10ul)
- Micropipet (2ul)
- Micropipet tips
Formamide Concentrations
- 0.5ul formamide + 49.5ul sterile water
- 1.0ul formamide + 49.0ul sterile water
- 1.5ul formamide + 48.5ul sterile water
- 2.0ul formamide + 48.0ul sterile water
- 2.5ul formamide + 47.5ul sterile water
- 3.0ul formamide + 47.0ul sterile water
- 3.5ul formamide + 46.5ul sterile water
- 4.0ul formamide + 46.0ul sterile water
- 4.5ul formamide + 45.5ul sterile water
- 5.0ul formamide + 45.0ul sterile water
- 5.5ul formamide + 44.5ul sterile water
- 0.0ul formamide + 50.0ul sterile water
Procedure
- Obtain the neat formamide (refrigerated) and sterile water
- Obtain and label 12 PCR tubes 1-12
- Obtain one more PCR tube to transfer 33ul of neat formamide inside, can put excess formamide in
- Using the 13th PCR tube, pipet appropriate amount of formamide and sterile water into the labeled PCR tubes according to number
- In a sterile environment, pipet the solution (50ul) from the PCR tube into the well cell
- Vortex the yeast culture briefly to resuspend the yeast cells, and then pipet 50ul of the wild yeast cells into each well in addition to the formamide-water solution
- Set up the plate reader as follows:
- Temperature: 30 degrees Celsius
- Mode: kinetic
- Wavelength: 600 nm
- Interval: 5 minutes
- Total run time: 24 hours
- Shake before read: 30 seconds
- Transfer the assay plate to the reader and read for 24 hours
- Record data
Results
- The x-axis is in time (min) and the y-axis is optical density at 600
- The x-axis corresponds to the concentrations listed up above under the title "formamide concentrations"
- 0% is an experimental error- since it has a concentration of 0% formamide anyway, it was left in the final graph
- The x-axis is in time (min) and the y-axis is optical density at 600
- The 3% formamide used in the pilot experiment and represented as the light blue color (in the top-most picture) was chosen to be used on the transformed yeast cells
- This was deduced due to the position of the yeast cells under 3% formamide stress
- Under 3% formamide, the yeast cells were able to grow, but didn't grow too much- simply put, the 3% line was right in the middle of the graph
Knockout Yeast Cell Gene
Materials / Equipment
- Neat formamide, 18ul
- Sterile water, 423ul
- 0.2ml flat cap PCR tube, 10 tubes
- Well plate
- Knock out yeast cells
- Molecular Devices SpectraMax Plus 384 Microplate Reader
- Micropipet (100ul)
- Micropipet (20ul)
- Micropipet (2ul)
- Micropipet tips
Procedure
- Obtain neat formamide (refrigerated) and sterile water
- Obtain and label PCR tubes 1-9
- Pipet approximately 18ul formamide into a 10th PCR tube, can put excess formamide in
- Using the 10th PCR tube, pipet 3.0ul of formamide into each PCR tube (labeled 1-9)
- Pipet 47.0ul of sterile water into each of the numbered PCR tubes
- In a sterile environment, pipet 50ul of formamide-water solution from the PCR tubes into well cells
- Vortex each yeast culture strain briefly to resuspend the cells, and then pipet 50ul of each knockout strain into the appropriate well cells in addition to the solution
- Set up the plate reader as follows:
- Temperature: 30 degrees Celsius
- Mode: kinetic
- Wavelength: 600 nm
- Interval: 5 minutes
- Total run time: 24 hours
- Shake before read: 30 seconds
- Transfer the assay plate to the reader and read for 24 hours
- Record data
Results
- The x-axis is in time (min) and the y-axis is optical density at 600
- Ran three trials, trial one through three is posted up above in picture form as a line graph
- The dark line in the middle of trail 2 and 3 is a scanning error from the Molecular Devices SpectraMax Plus 384 Microplate Reader
- Computed doubling times from the average times of the three trials for further analyzation