UW-Stout/pH FA21
Contents
Introduction
We will be stressing our yeast by exposing it to different pH and measuring its sensitivity based on its survival rate. In this experiment, we will be using a citric acid buffer of 7, 6, 5, 4, 3 , 2.6, 2.4, and 2.2 pH as a calibration experiment for the first 12 to 15 hours on a wild type strain.
Materials
- Micropipette
- Transformed yeast
- De-ionized Water
- Citric acid
- Disodium phosphate
- 3-15mL tube
- 3-Micron filter
- 3-sterile syringes
- Scale
- Corning COSTAR 96-well clear flat-bottom assay plate
- Falcon round-bottom polypropylene tubes
- YPD media, both broth and 2% agar plates
- Micropipette tips
- 6 PCR tubes containing wild type yeast and 5 knockout strains
- Sterile Water
- 1.5mL microcentrifuge tubes
- Incubator set to 30°C.
- New Brunswick TC-7 Tissue Culture Roller Drum
- Molecular Devices SpectraMax Plus 384 Microplate Reader
Procedure
Preparing Yeast
Three days before the experiment, streak knockout strains onto YPD plates. The morning before the experiment, pick a colony from each strain into 5 ml YPD broth in a disposable test-tube. Incubate on the roller drum at 30°C for 8-10 hours. The evening before the experiment, transfer 20 ul of each strain into 5 ml of fresh YPD broth. Incubate on a roller drum at 30°C overnight. The morning of the experiment, dilute each culture to an OD600 of 0.1-0.2 in YPD broth. Return to the drum roller until ready to test.
Preparing Buffer Stock Solutions
- Retrieve your citric acid and disodium phosphate
- Determine the amount of each needed to make 10mL of .10M stock solutions of disodium phosphate and citric acid.
- From those stock solutions, buffer solutions can be made
- Thoroughly mix solutions to ensure all solids are dissolved
Sterilization
- Acquire 3-15mL sterile syringes, 3-micron filter adapters for your syringes, and 3 new 15mL tubes.
- Attach micron filter to tip of syringe and remove plunger
- Pour first stock solution into syringe
- While holding syringe over UNUSED 15mL tube, insert plunger and push your solution through filter into tube, seal and label
- Repeat with second stock solution and DI water
Preparing Buffer Solutions
pH 7
- Using a micropipette, pipet 329.4uL .10M disodium phosphate solution into microcentrifuge tube
- Add 35.3uL .1M citric acid solution into same tube
- Add 635.3uL sterile water into tube
- seal tube, vortex, and label pH 7
pH 6
- pipet 252.6uL disodium phosphate solution into microcentrifuge tube
- Add 73.7uL citric acid solution into same tube
- Add 673.7uL sterile water into tube
- seal tube, vortex, and label pH 6
pH 5
- pipet 206uL disodium phosphate solution into microcentrifuge tube
- Add 97uL citric acid solution into same tube
- Add 697uL sterile water into tube
- seal tube, vortex, and label pH 5
pH 4
- pipet 154.2uL disodium phosphate solution into microcentrifuge tube
- Add 122.9uL citric acid solution into same tube
- Add 722.9uL sterile water into tube
- seal tube, vortex, and label pH 4
pH 3
- pipet 82.2uL disodium phosphate solution into microcentrifuge tube
- Add 158.9uL citric acid solution into same tube
- Add 758.9uL sterile water into tube
- seal tube, vortex, and label pH 3
pH 2.6
- pipet 43.6uL disodium phosphate solution into microcentrifuge tube
- Add 178.2uL citric acid solution into same tube
- Add 778.2uL sterile water into tube
- seal tube, vortex, and label pH 2.6
pH 2.4
- pipet 24.8uL disodium phosphate solution into microcentrifuge tube
- Add 187.6uL citric acid solution into same tube
- Add 787.6uL sterile water into tube
- seal tube, vortex, and label pH 2.4
pH 2.2
- pipet 8uL disodium phosphate solution into microcentrifuge tube
- Add 196uL citric acid solution into same tube
- Add 796uL sterile water into tube
- seal tube, vortex, and label pH 2.2
Addition of Buffer to Yeast
- Vortex Yeast cultures
- Add 50uL yeast cells in each well for DI water and each pH level
- Add 50uL of DI water to first well as a control
- add 50uL pH buffer to each subsequent well, descending in pH level
Obtaining Growth Curve Data/Preparing Plate Reader
Preparing Plate Reader
- Temperature: at 30 degrees Celsius
- Mode: Kinetic
- Read: 600 n
- Intervals: 5 minutes
- Total run time: 24 hours
- Shake before read: 30 seconds
- Transfer the assay plate to the reader and run for 24 hours
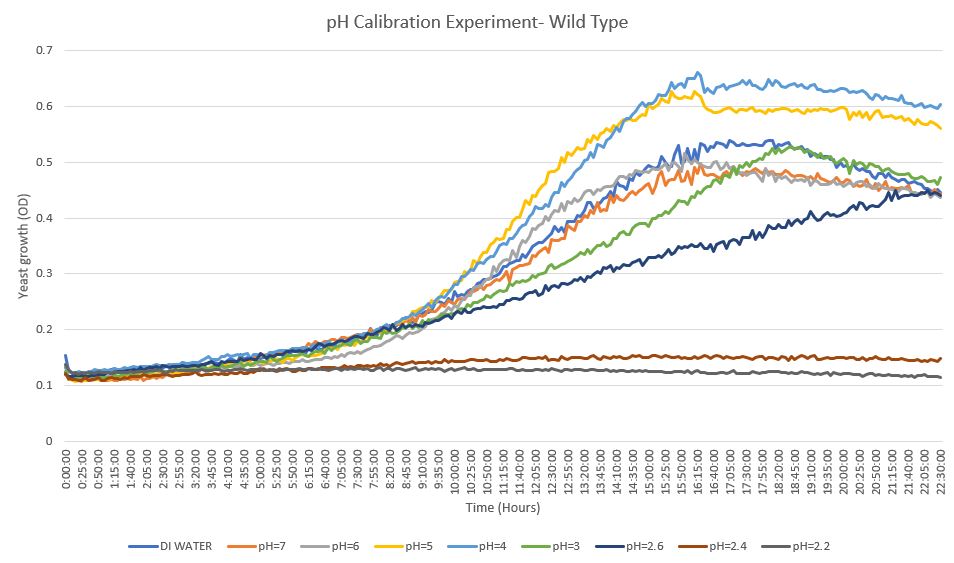
RESULTS: GROWTH CURVE FOR CALIBRATION EXPERIMENT
Notes
From our calibration experiment, we used each of our pH buffers in order to see how much we could stress the wild type (BY4735) without it dying. Starting with DI water and ending with the pH of 2.2, we saw that none of the pH levels killed off the yeast. However, we noticed that pH of 2.6 provided a desirable amount of stress without stunting growth significantly.
Final Protocol
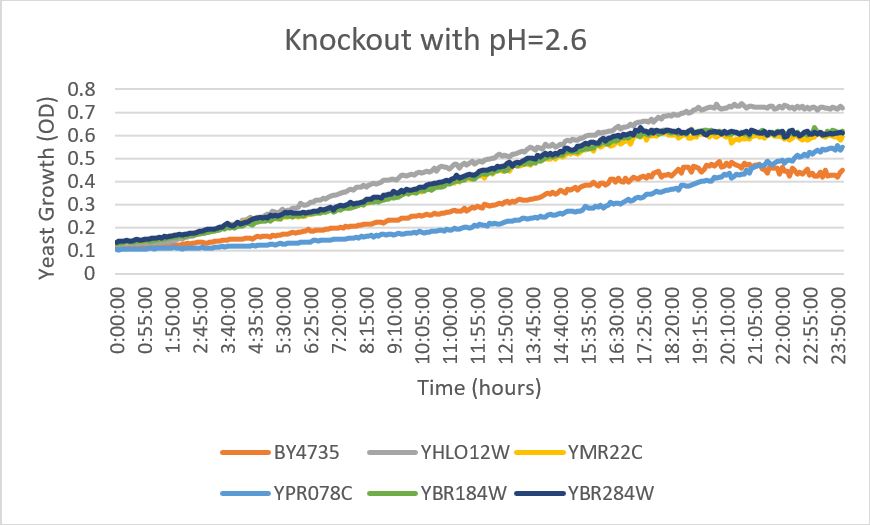
- REPEAT PILOT PROTOCOL USING ONLY pH 2.6
- Use the Wild Type strain, and the knockout strains
- repeat "preparing plate reader"
- Annotate graph curve